
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.56QP
Fumaric acid, C4H4O4, occurs in the
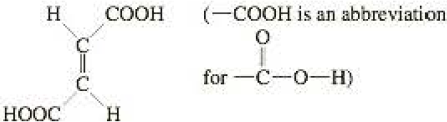
Maleic acid is the cis isomer of fumaric add. Using
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the proper IUPAC name only for the following
compound. Dashes, commas, and spaces must be used
correctly, but do not use italics in Canvas.
The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 M
What is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)
Chapter 10 Solutions
General Chemistry - Standalone book (MindTap Course List)
Ch. 10.1 - An atom in a molecule is surrounded by four pairs...Ch. 10.1 - Use the VSEPR method to predict the geometry of...Ch. 10.1 - Prob. 10.2ECh. 10.2 - Bromine trifluoride, BrF3, has a nonzero dipole...Ch. 10.2 - Which of the following would be expected to have a...Ch. 10.2 - Two molecules, each with the general formula AX3,...Ch. 10.3 - Using hybrid orbitals, describe the bonding in NH3...Ch. 10.4 - Describe the bonding on the carbon atom in carbon...Ch. 10.4 - Dinitrogen difluoride (see Example 10.5) exists as...Ch. 10.4 - Prob. 10.3CC
Ch. 10.6 - The C2 molecule exists in the vapor phase over...Ch. 10.6 - Give the orbital diagram and electron...Ch. 10 - Describe the main features of the VSEPR model.Ch. 10 - According to the VSEPR model, what are the...Ch. 10 - Why is a lone pair expected to occupy an...Ch. 10 - Prob. 10.4QPCh. 10 - Explain why nitrogen trifluoride has a small...Ch. 10 - Prob. 10.6QPCh. 10 - What is the angle between two sp3 hybrid orbitals?Ch. 10 - Prob. 10.8QPCh. 10 - Prob. 10.9QPCh. 10 - How does the valence bond description of a...Ch. 10 - Prob. 10.11QPCh. 10 - What factors determine the strength of interaction...Ch. 10 - Prob. 10.13QPCh. 10 - Prob. 10.14QPCh. 10 - Prob. 10.15QPCh. 10 - Describe the bonding in O3, using molecular...Ch. 10 - Prob. 10.17QPCh. 10 - Which of the following molecular geometries does...Ch. 10 - Which of the following would be a polar molecule?...Ch. 10 - Prob. 10.20QPCh. 10 - Best Lewis Formula and Molecular Geometry A...Ch. 10 - Prob. 10.22QPCh. 10 - Prob. 10.23QPCh. 10 - Which of the following molecular models correctly...Ch. 10 - Prob. 10.25QPCh. 10 - Prob. 10.26QPCh. 10 - Indicate what hybrid orbital depicted below is...Ch. 10 - An atom in a molecule has two bonds to other atoms...Ch. 10 - Two compounds have the same molecular formula,...Ch. 10 - A neutral molecule is identified as a...Ch. 10 - Acetic acid, the sour constituent of vinegar, has...Ch. 10 - What are the bond angles predicted by the VSEPR...Ch. 10 - Predict the shape or geometry of the following...Ch. 10 - Use the electron-pair repulsion model to predict...Ch. 10 - Predict the geometry of the following ions, using...Ch. 10 - Use the VSEPR model to predict the geometry of the...Ch. 10 - For each of the following molecules, state the...Ch. 10 - For each of the following molecules, state the...Ch. 10 - Prob. 10.39QPCh. 10 - From the electron-pair repulsion model, predict...Ch. 10 - Predict the geometries of the following ions,...Ch. 10 - Name the geometries expected for the following...Ch. 10 - a The molecule AsF3 has a dipole moment of 2.59 D....Ch. 10 - a The molecule BrF3 has a dipole moment of 1.19 D....Ch. 10 - Which of the following molecules would be expected...Ch. 10 - Which of the following molecules would be expected...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - a Mercury(II) chloride dissolves in water to give...Ch. 10 - a Nitrogen trifluoride, NF3, is a relatively...Ch. 10 - a Carbonyl fluoride, COF2, is an extremely...Ch. 10 - a The molecule HNNH exists as a transient species...Ch. 10 - The hyponitrite ion, ONNO, exists in solid...Ch. 10 - Fumaric acid, C4H4O4, occurs in the metabolism of...Ch. 10 - Describe the electronic structure of each of the...Ch. 10 - Use molecular orbital theory to describe the...Ch. 10 - Prob. 10.59QPCh. 10 - Write the molecular orbital configuration of the...Ch. 10 - Predict the molecular geometry of the following: a...Ch. 10 - Prob. 10.62QPCh. 10 - Which of the following molecules or ions are...Ch. 10 - Which of the following molecules or ions are...Ch. 10 - Describe the hybrid orbitals used by each carbon...Ch. 10 - Prob. 10.66QPCh. 10 - Explain how the dipole moment could be used to...Ch. 10 - Two compounds have the formula Pt(NH3)2Cl2....Ch. 10 - Explain in terms of bonding theory why all four...Ch. 10 - Explain in terms of bonding theory why all atoms...Ch. 10 - What is the molecular orbital configuration of...Ch. 10 - Prob. 10.72QPCh. 10 - Calcium carbide, CaC2, consists of Ca2+ and C22...Ch. 10 - Sodium peroxide, Na2O2, consists of Na+ and O22...Ch. 10 - The oxygen oxygen bond in O2 is 112 pm and in O2...Ch. 10 - The nitrogennitrogen bond distance in N2 is 109...Ch. 10 - Using molecular orbital theory, determine the...Ch. 10 - The ionization energy of O2 is smaller than the...Ch. 10 - Prob. 10.79QPCh. 10 - Prob. 10.80QPCh. 10 - Prob. 10.81QPCh. 10 - Prob. 10.82QPCh. 10 - What is the biological importance of stratospheric...Ch. 10 - Prob. 10.84QPCh. 10 - Prob. 10.85QPCh. 10 - The bond length in C2 is 131 pm. Compare this with...Ch. 10 - Calcium carbide, CaC2, has an ionic structure with...Ch. 10 - Write Lewis formulas for the BF molecule (two with...Ch. 10 - Boron trifluoride, BF3, reacts with ammonia, NH3,...Ch. 10 - Prob. 10.90QPCh. 10 - Allene (1,2-propadicne), a gas, has the following...Ch. 10 - Prob. 10.92QPCh. 10 - The triiodide ion, I3, and the azide ion, N3, have...Ch. 10 - Hydrogen azide (also known as hydrazoic acid),...Ch. 10 - Prob. 10.95QPCh. 10 - A molecule XF6 (having no lone pairs) has a dipole...Ch. 10 - Describe the molecular orbital configurations of...Ch. 10 - Prob. 10.98QPCh. 10 - Three different compounds have the same molecular...Ch. 10 - Prob. 10.100QPCh. 10 - Prob. 10.101QPCh. 10 - Solid sulfur normally consists of crystals of S8...Ch. 10 - Prob. 10.103QPCh. 10 - Consider the bonding in nitrate ion, NO3. First...Ch. 10 - A molecular compound is composed of 52.5% Xe,...Ch. 10 - A molecular compound is composed of 58.8% Xe,...Ch. 10 - A compound of chlorine and fluorine. ClFn, reacts...Ch. 10 - Excess fluorine, F2(g), reacts at 150C with...Ch. 10 - Prob. 10.109QPCh. 10 - One resonance formula of benzene, C6H6, is What is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardThe reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardWhat is the name of the major product formed during the reaction between benzoyl chloride and phenol? benzyl ester O phenyl benzoate ○ cyclopentanoate ○ benzyl phenoate ○ benzenecarboxylic acidarrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly.arrow_forward
- Provide the proper IUPAC name (only) for the following compound. Dashes, commas, and spaces must be used correctly. HO. OHarrow_forwardQuestion 2 0/1 pts Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly. HO CH 3 1-methyl-1-cyclohexanecarboxylic acidarrow_forwardPlease assign all the carbons for C-NMR and hydrogen for H-NMR. Please if I can get that less than hourarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY