
(a)
Interpretation:
The product expected when
Concept introduction:
The nucleophilic substitution reactions are the reactions in which a group is substituted by an nucleophile. The
The
Answer to Problem 10.39AP
The product obtained on the reaction of
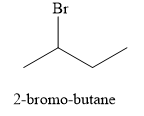
Explanation of Solution
The product obtained on the reaction of
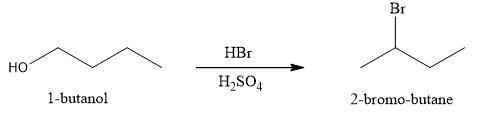
Figure 1
The alcohol
The product obtained on the reaction of
(b)
Interpretation:
The product expected when
Concept introduction:
An
Answer to Problem 10.39AP
The product obtained on the reaction of
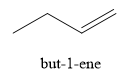
Explanation of Solution
The product obtained on the reaction of
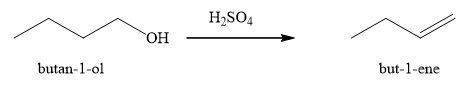
Figure 2
The alcohol
The product obtained on the reaction of
(c)
Interpretation:
The product expected when
is to be stated.
Concept introduction:
Primary and secondary alcohols can be oxidized into
Answer to Problem 10.39AP
The products obtained on the reaction of
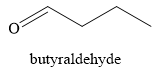
Explanation of Solution
The products obtained on the reaction of
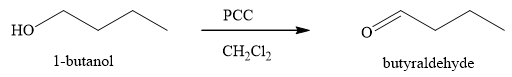
Figure 3
Primary alcohols on oxidation give aldehyde. Aldehydes can further undergo oxidation to give
The products obtained on the reaction of
(d)
Interpretation:
The product expected when
Concept introduction:
Acid-base reaction are among the fastest reaction in the chemistry. Acids and bases react vigorously generating heat and water normally. Metals are basic in nature due to the presence of free electrons to donate. Alcohols are both acidic and basic in nature.
Answer to Problem 10.39AP
The product obtained on the reaction of
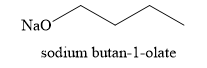
Explanation of Solution
The product obtained on the reaction of
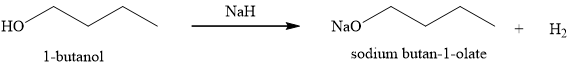
Figure 4
The sodium hydride is a very strong base and it undergoes acid-base reaction with alcohol
The product obtained on the reaction of
(e)
Interpretation:
The product expected when the product of part (d) is reacted with
Concept introduction:
The nucleophilic substitution reactions are the reactions in which a group is substituted by an nucleophile. The rate of reaction depends upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.39AP
The product obtained on the reaction of sodium
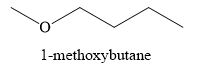
Explanation of Solution
The product of part (d) is sodium
The product obtained on the reaction of sodium
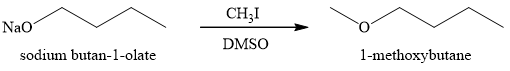
Figure 5
The sodium
The product obtained on the reaction of sodium
(f)
Interpretation:
The product expected when
Concept introduction:
The hydroxide group in alcohols is not a good leaving group in order to perform a nucleophilic substitution reaction on alcohols to produce more compounds. Hydroxide group is made a good leaving group with the help of some compounds like methanesulfonyl chloride and p-toluenesulfonyl chloride.
Answer to Problem 10.39AP
The product obtained on the reaction of
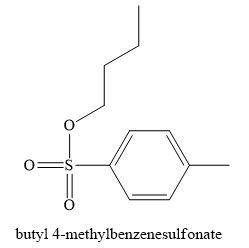
Explanation of Solution
The product obtained on the reaction of
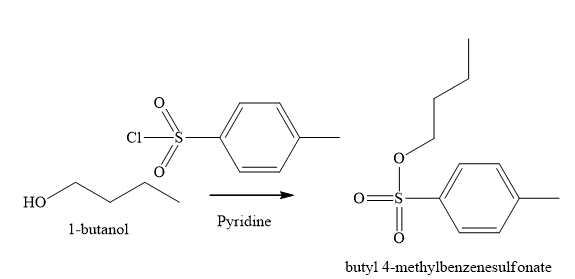
Figure 6
The reaction of an alcohol with sulfonate derivatives such as methanesulfonyl chloride and
The product obtained on the reaction of
(g)
Interpretation:
The product expected when
Concept introduction:
The reaction of an
Answer to Problem 10.39AP
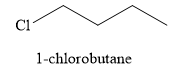
The product obtained on the reaction of
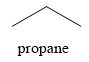
Explanation of Solution
The product obtained on the reaction of
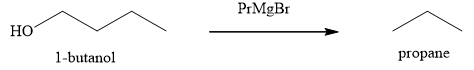
Figure 7
The
The product obtained on the reaction of
(h)
Interpretation:
The product expected when
Concept introduction:
The nucleophilic substitution reactions are the reactions in which a group is substituted by an nucleophile. The rate of reaction depends upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.39AP
The product obtained on the reaction of
Explanation of Solution
The product obtained on the reaction of
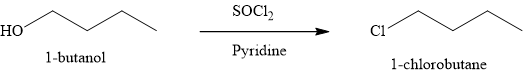
Figure 8
The alcohol
The product obtained on the reaction of
(i)
Interpretation:
The product expected when
Concept introduction:
The nucleophilic substitution reactions are the reactions in which a group is substituted by an nucleophile. The rate of reaction depends upon the nucleophilicity and concentration of the nucleophile.
The
Answer to Problem 10.39AP
The product obtained on the reaction of
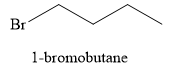
Explanation of Solution
The product obtained on the reaction of
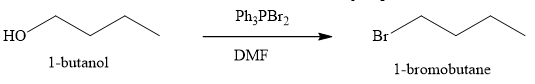
Figure 9
The alcohol
The product obtained on the reaction of
(j)
Interpretation:
The product expected when product of part (a) is reacted with
Concept introduction:
The reaction of an alkyl halide with a metal like magnesium in the presence of dry ether leads to the formation of
Answer to Problem 10.39AP
The product obtained on reaction of
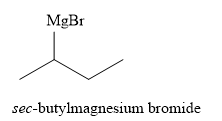
Explanation of Solution
The product of part (a) is
The product obtained on reaction of
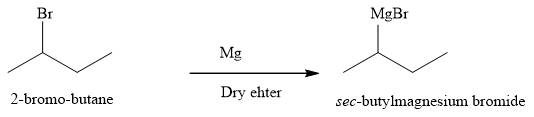
Figure 10
The alkyl halide reacts with magnesium metal in dry ether to produce the Grignard reagent. The
The product obtained on reaction of
(k)
Interpretation:
The product expected when the product of part (f) is reacted with
Concept introduction:
An
Answer to Problem 10.39AP
The product obtained on the reaction of
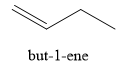
Explanation of Solution
The product of part (f) is
The product obtained on the reaction of
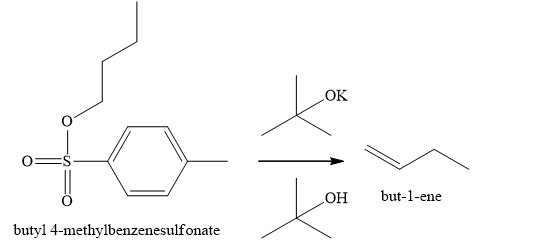
Figure 11
The ester
The product obtained on the reaction of
(l)
Interpretation:
The product expected when
Concept introduction:
The hydroxide group in alcohols is not a good leaving group. In order to perform a nucleophilic substitution reaction on alcohols to produce more compounds hydroxide group is made a good leaving group with the help of some compounds like methanesulfonyl chloride and
Answer to Problem 10.39AP
The product obtained when
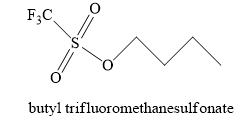
Explanation of Solution
The product obtained when
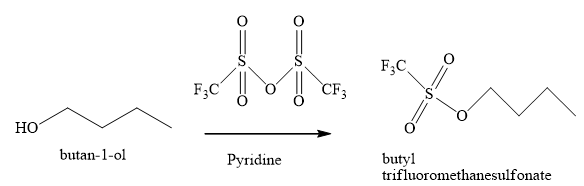
Figure 12
The triflic anhydride is also one of the compounds used to make hydroxide group a good leaving group. This compound is used much more reactive ester as compared to given by tosylates and mesylates. The yield of the product obtained by this ester of substitution is very high.
The
The product obtained when
(m)
Interpretation:
The product expected when the product of (l) is reacted with anhydrous
Concept introduction:
The hydroxide group in alcohols is not a good leaving group in order to perform a nucleophilic substitution reaction on alcohols to produce more compounds. Hydroxide group is made a good leaving group with the help of some compounds like methanesulfonyl chloride and
Answer to Problem 10.39AP
The product obtained when butyl trifluoromethanesulfonate is reacted with anhydrous
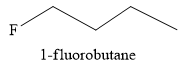
Explanation of Solution
The product obtained when butyl trifluoromethanesulfonate is reacted with anhydrous
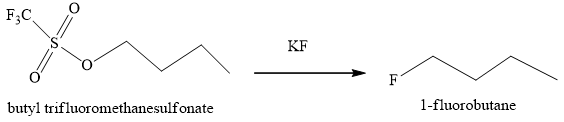
Figure 13
The compound butyl trifluoromethanesulfonate undergoes nucleophilic substitution reaction with
The product obtained when butyl trifluoromethanesulfonate is reacted with anhydrous
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry Study Guide and Solutions
- answer with mechanisms and steps. handwritten please!arrow_forwardHello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forwardTrue or false, chemistryarrow_forward
- answer thse questions with mechanisms and steps. handwritten please!arrow_forwardC app.aktiv.com Draw the product of the following reaction sequence. Ignore any inorganic byproducts formed. H O 1. (CH3CH2)2CuLi, THF 2. CH3Br Drawingarrow_forwardDraw the product of the following reaction sequence. Ignore any inorganic byproducts formed. H O 1. (CH3CH2)2CuLi, THF 2. CHзBr Drawingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





