
Concept explainers
(a)
Interpretation:
Line formula for given
(a)
Explanation of Solution
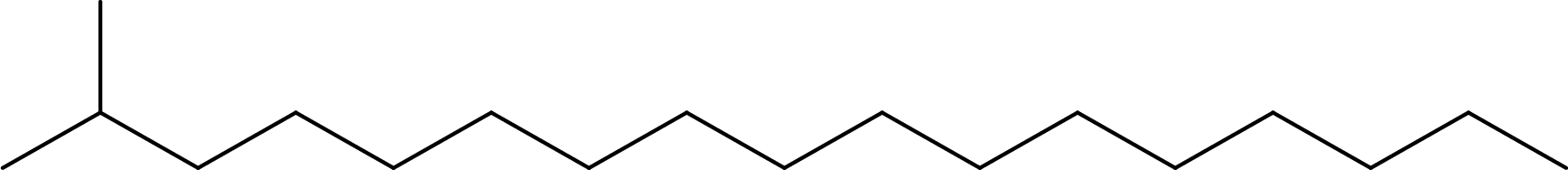
Line formula for 2-methylheptadecane:
From the given name, the parent alkane is found to be heptadecane. Substituent that is present is a methyl group on carbon-2. Line formula for 2-methylheptadecane can be drawn as,
Line formula for 17,21-dimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are two methyl groups on carbon-17 and carbon-21. Line formula for 17,21-dimethylheptatriacontane can be drawn as,

Line formula for 15,19-dimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are two methyl groups on carbon-15 and carbon-19. Line formula for 15,19-dimethylheptatriacontane can be drawn as,

Line formula for 15,19,23-trimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are three methyl groups on carbon-15, carbon-19 and carbon-23. Line formula for 15,19,23-trimethylheptatriacontane can be drawn as,

(c)
Interpretation:
Molar mass of given alkanes has to be calculated..
(c)
Explanation of Solution
Molar mass of 2-methylheptadecane:
Molecular formula of 2-methylheptadecane is given as
Molar mass of 2-methylheptadecane is calculated as shown below,
Therefore, molar mass of 2-methylheptadecane is
Molar mass of 17,21-dimethylheptatriacontane:
Molecular formula of 17,21-dimethylheptatriacontane is given as
Molar mass of 17,21-dimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 17,21-dimethylheptatriacontane is
Molar mass of 15,19-dimethylheptatriacontane:
Molecular formula of 15,19-dimethylheptatriacontane is given as
Molar mass of 15,19-dimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 15,19-dimethylheptatriacontane is
Molar mass of 15,19,23-trimethylheptatriacontane:
Molecular formula of 15,19,23-trimethylheptatriacontane is given as
Molar mass of 15,19,23-trimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 15,19,23-dimethylheptatriacontane is
Want to see more full solutions like this?
Chapter 10 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY-ALEKS 360
- please help by Draw the following structures (Lewis or line-angle drawing).arrow_forwardplease helparrow_forwardConsider the reaction: 2 A (aq) ⇌ B(aq) Given the following KC values and starting with the initial concentration of A = 4.00 M, complete ICE diagram(s)and find the equilibrium concentrations for A and B.A) KC = 4.00B) KC = 200C) KC = 8.00 x10-3arrow_forward
- 5) Consider the reaction: Cl2 (g) + F2 (g) ⟷ 2 ClF (g) KP=? The partial pressure of 203 kPa for Cl2 and a partial pressure of 405 kPa for F2. Upon reaching equilibrium, thepartial pressure of ClF is 180 kPa. Calculate the equilibrium concentrations and then find the value for KP.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception tothe general ionization energy (IE) trend. For the two elements involved, answer the followingquestions. Be sure to cite sources for all physical data that you use.a. (2 pts) Identify the two elements and write their electronic configurations.b. (2 pts) Based on their configurations, propose a reason for the IE trend exception.c. (5 pts) Calculate effective nuclear charges for the last electron in each element and theAllred-Rochow electronegativity values for the two elements. Can any of these valuesexplain the IE trend exception? Explain how (not) – include a description of how IErelates to electronegativity.arrow_forward
- Explain what characteristics of metalloids are more like metals and which are more like nonmetals, based on Na, Mg, Fe, Cl, and Ar.arrow_forwardplease solve this, and help me know which boxes to check. Thank you so much in advance.arrow_forwardElectronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Describe how electronegativity is illustrated on the periodic table including trends between groups and periods and significance of atom size.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





