
Concept explainers
(a)
Interpretation:
Line formula for given
(a)
Explanation of Solution
Line formula for 2-methylheptadecane:
From the given name, the parent alkane is found to be heptadecane. Substituent that is present is a methyl group on carbon-2. Line formula for 2-methylheptadecane can be drawn as,
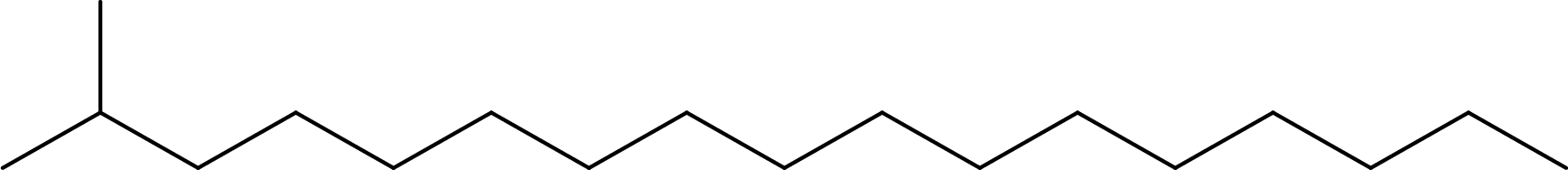
Line formula for 17,21-dimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are two methyl groups on carbon-17 and carbon-21. Line formula for 17,21-dimethylheptatriacontane can be drawn as,

Line formula for 15,19-dimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are two methyl groups on carbon-15 and carbon-19. Line formula for 15,19-dimethylheptatriacontane can be drawn as,

Line formula for 15,19,23-trimethylheptatriacontane:
From the given name, the parent alkane is found to be heptatriacontane. Substituent that is present are three methyl groups on carbon-15, carbon-19 and carbon-23. Line formula for 15,19,23-trimethylheptatriacontane can be drawn as,

(c)
Interpretation:
Molar mass of given alkanes has to be calculated..
(c)
Explanation of Solution
Molar mass of 2-methylheptadecane:
Molecular formula of 2-methylheptadecane is given as
Molar mass of 2-methylheptadecane is calculated as shown below,
Therefore, molar mass of 2-methylheptadecane is
Molar mass of 17,21-dimethylheptatriacontane:
Molecular formula of 17,21-dimethylheptatriacontane is given as
Molar mass of 17,21-dimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 17,21-dimethylheptatriacontane is
Molar mass of 15,19-dimethylheptatriacontane:
Molecular formula of 15,19-dimethylheptatriacontane is given as
Molar mass of 15,19-dimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 15,19-dimethylheptatriacontane is
Molar mass of 15,19,23-trimethylheptatriacontane:
Molecular formula of 15,19,23-trimethylheptatriacontane is given as
Molar mass of 15,19,23-trimethylheptatriacontane is calculated as shown below,
Therefore, molar mass of 15,19,23-dimethylheptatriacontane is
Want to see more full solutions like this?
Chapter 10 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY
- What is the product of the reaction? F3C. CF3 OMe NaOH / H₂Oarrow_forwardWhat would you expect to be the major product obtained from the following reaction? Please explain what is happening here. Provide a detailed explanation and a drawing showing how the reaction occurs. The correct answer to this question is V.arrow_forwardPlease answer the question for the reactions, thank youarrow_forward
- What is the product of the following reaction? Please include a detailed explanation of what is happening in this question. Include a drawing showing how the reagent is reacting with the catalyst to produce the correct product. The correct answer is IV.arrow_forwardPlease complete the reactions, thank youarrow_forwardConsider the synthesis. What is compound Y? Please explain what is happening in this question. Provide a detailed explanation and a drawing to show how the compound Y creates the product. The correct answer is D.arrow_forward
- What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.arrow_forwardWhat is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.arrow_forwardWhat is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





