
Concept explainers
(a)
Interpretation:
Monobromination products obtained when propane treated with bromine has to given and the percentage of the monobromo products has to be expected.
Concept Introduction:
Halogenation reaction is the one where atom or atoms of halogens get substituted in a carbon chain. Halogenation is a type of substitution reaction.
In
IUPAC rules for naming alkanes:
There are about five rules that has to be followed for naming an alkane and they are,
- The longest continuous carbon chain in the compound has to be identified. This is known as parent compound. From this the parent name is obtained. Suffix “–ane” (for alkane) is added at the end of the prefix which gives information about the number of carbon atoms.
- Numbering has to be done so that the lowest number is given to the first group that is encountered in the parent chain.
- Naming and numbering has to be given for each atom or group that is attached to the parent chain. Numbering has to be done in a way that substituents get the least numbering.
- If the same substitution is present in the parent chain more than once, a separate prefix is added which tells about the number of times the substituent occurs. Prefixes used are di-, tri-, tetra-, penta- etc.
- Name of the substituents has to be placed in an alphabetical order before the parent compound name.
(a)
Explanation of Solution
Propane on reaction with bromine in presence of light undergoes bromination. Two monobrominated products are obtained in case of propane. This can be represented as,
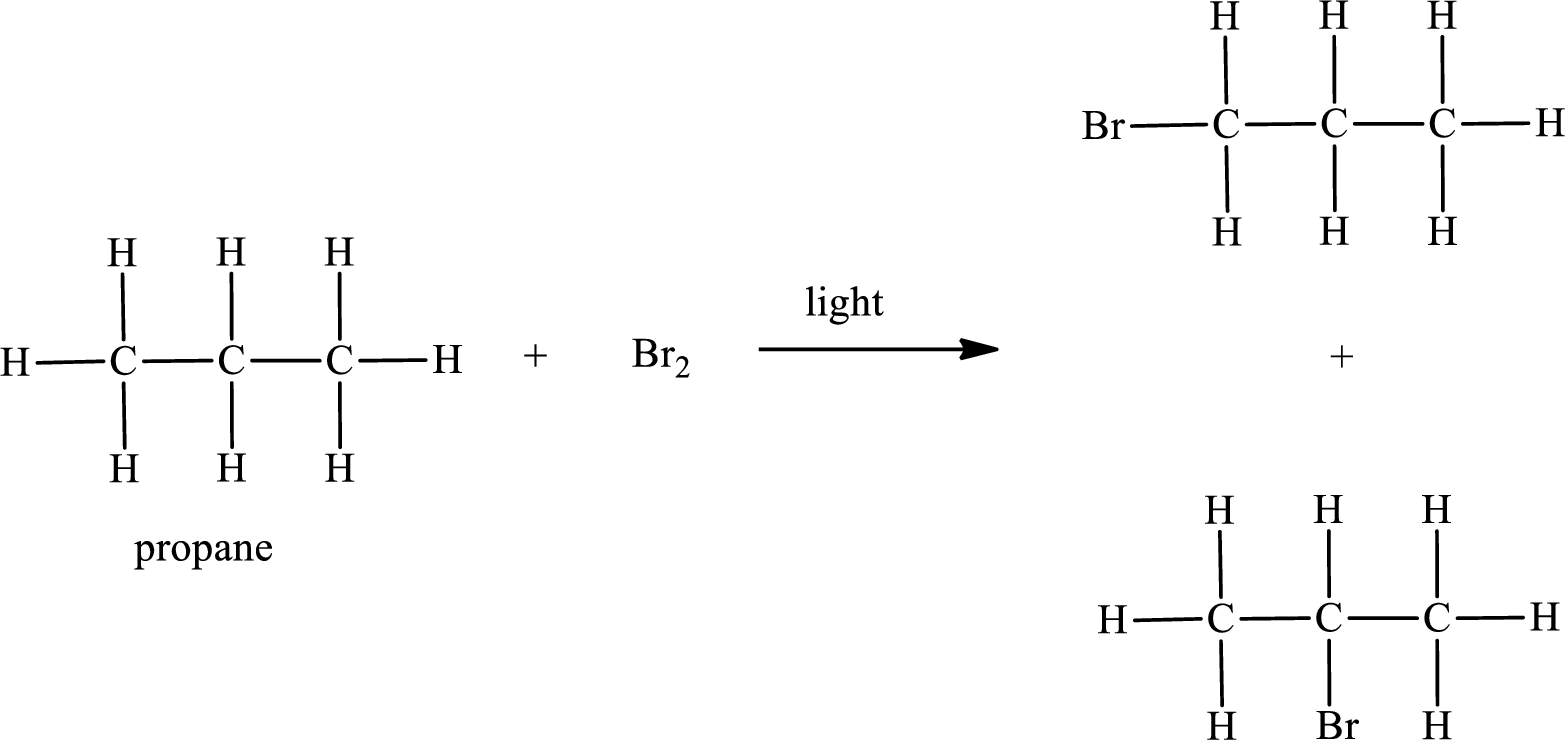
IUPAC names:
First monobromo derivative:
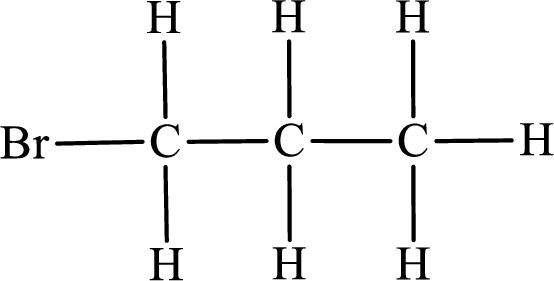
In the given compound, the longest carbon chain is found to contain three carbon atoms. Therefore, the parent alkane name is propane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
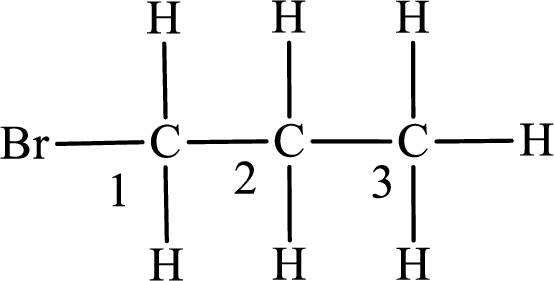
The substituent present in the given compound is a bromine atom. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
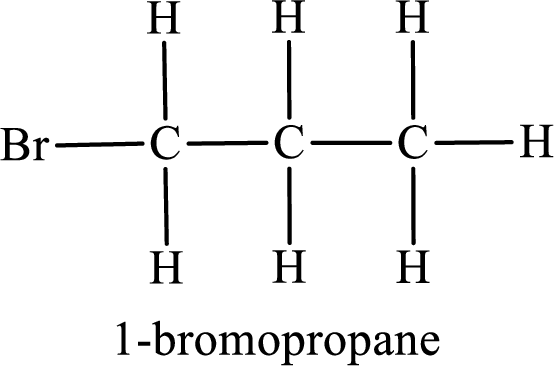
Parent chain is propane and the substituent present is 1-bromo. Hence, the IUPAC name is given as 1-bromopropane.
Second monobromo derivative:
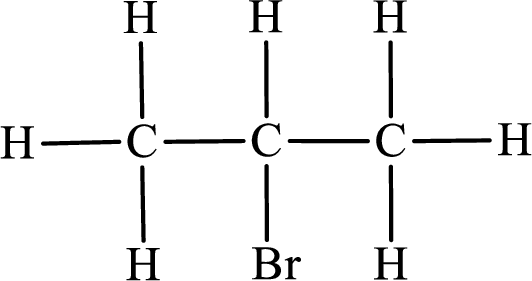
In the given compound, the longest carbon chain is found to contain three carbon atoms. Therefore, the parent alkane name is propane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
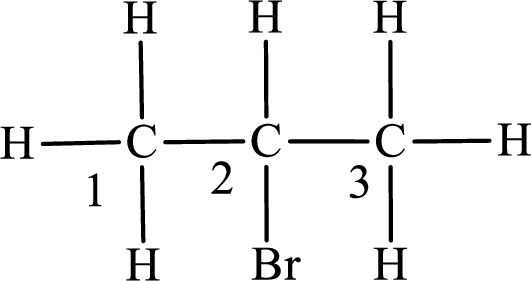
The substituent present in the given compound is a bromine atom. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
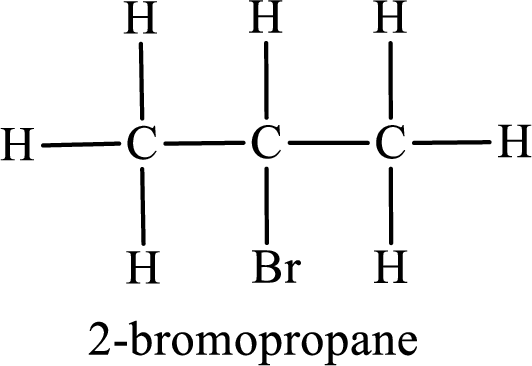
Parent chain is propane and the substituent present is 2-bromo. Hence, the IUPAC name is given as 2-bromopropane.
Percentage of monobrominated products formed:
Total number of hydrogen atoms in propane is eight. Six equivalents of hydrogen is required for the formation of 1-bromopropane and two equivalents of hydrogen is required for the formation of 2-bromopropane. Therefore, the ratio of formation of 1-bromopropane to 2-bromopropane can be given as
(b)
Interpretation:
Monobromination products obtained when 2-methylpropane treated with bromine has to given and the percentage of the monobromo products has to be expected.
Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
2-methylpropane on reaction with bromine in presence of light undergoes bromination. Two monobrominated products are obtained in case of propane. This can be represented as,
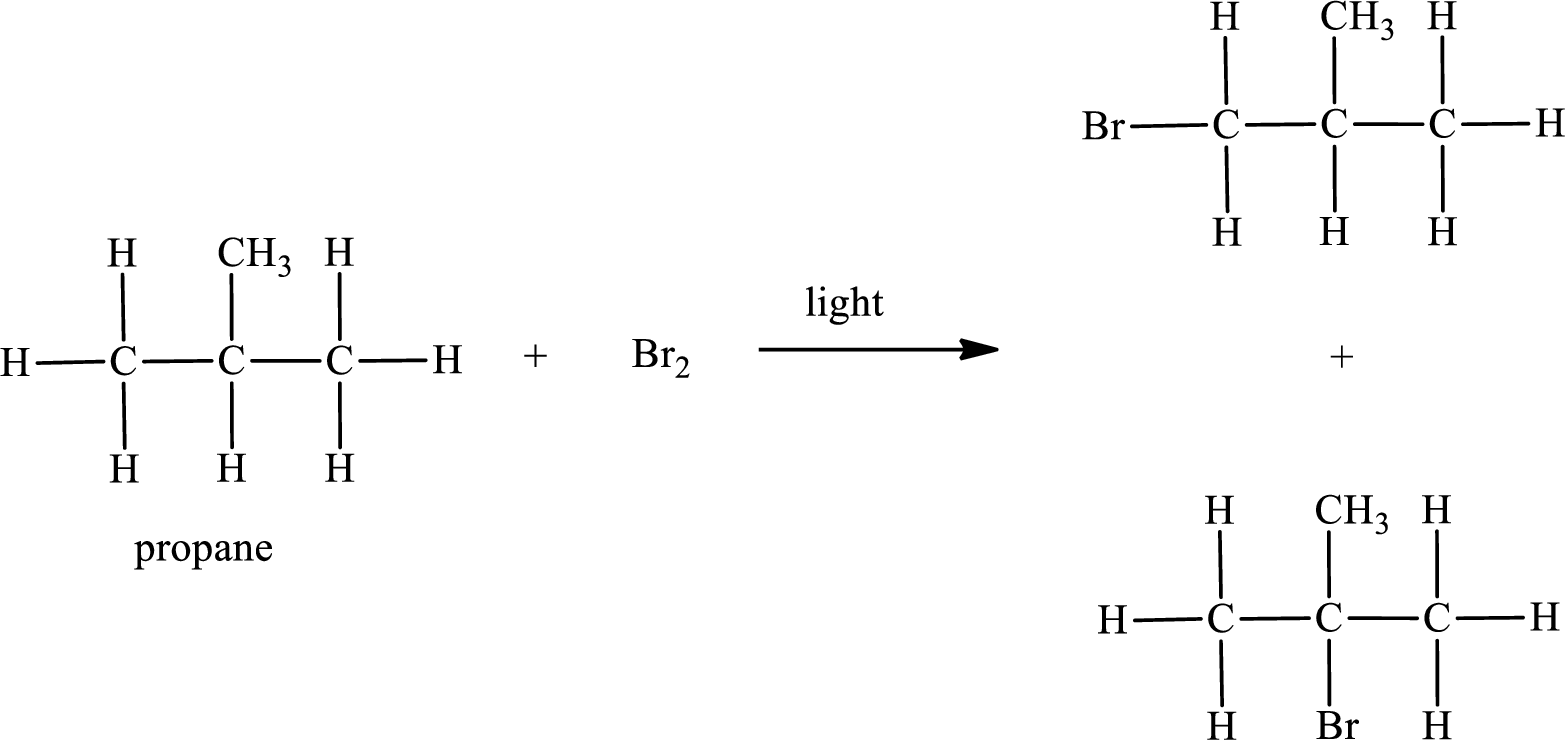
IUPAC names:
First monobromo derivative:
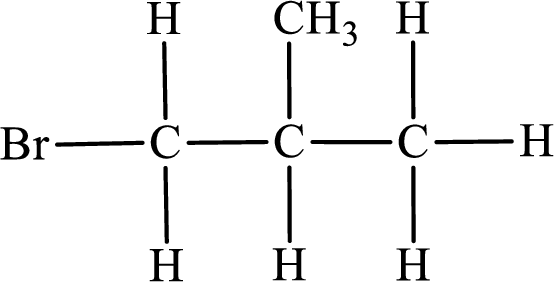
In the given compound, the longest carbon chain is found to contain three carbon atoms. Therefore, the parent alkane name is propane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
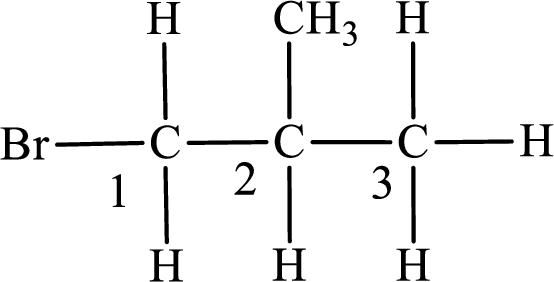
The substituent present in the given compound are a bromine atom and methyl group. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
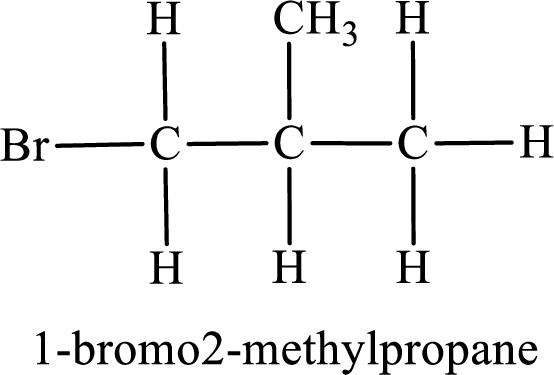
Parent chain is propane and the substituent present is 1-bromo-2-methyl. Hence, the IUPAC name is given as 1-bromo-2-methylpropane.
Second monobromo derivative:
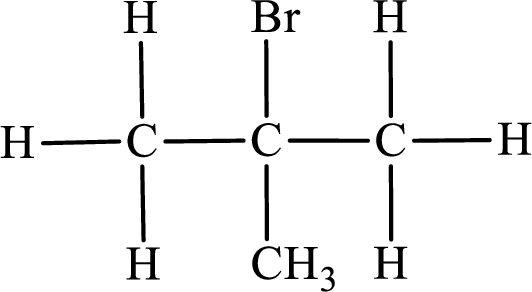
In the given compound, the longest carbon chain is found to contain three carbon atoms. Therefore, the parent alkane name is propane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
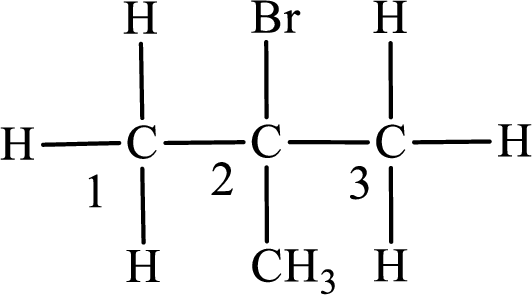
The substituent present in the given compound are a bromine atom and methyl group. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
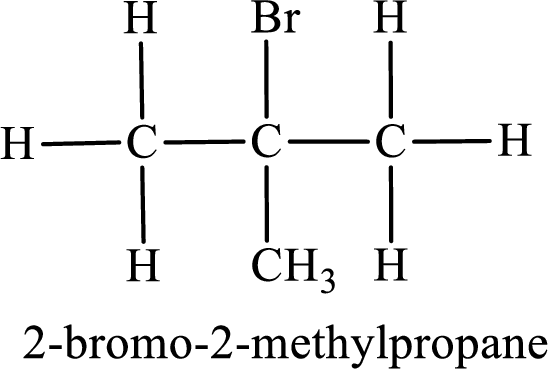
Parent chain is propane and the substituent present is 2-bromo-2-methyl. Hence, the IUPAC name is given as 2-bromo-2-methylpropane.
Percentage of monobrominated products formed:
Total number of hydrogen atoms in propane is ten. Nine equivalents of hydrogen is required for the formation of 1-bromo-2-methylpropane and on equivalents of hydrogen is required for the formation of 2-bromo-2-methyl propane. Therefore, the ratio of formation of 1-bromo-2-methyl propane to 2-bromo-2-methyl propane can be given as
Want to see more full solutions like this?
Chapter 10 Solutions
GENERAL,ORGANIC,+BIOCHEMISTRY
- Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCN is a weak acid. acids: 0.29 mol of NaOH is added to 1.0 L of a 1.2M HCN solution. bases: ☑ other: 0.09 mol of HCl is added to acids: 1.0 L of a solution that is bases: 0.3M in both HCN and KCN. other: 0,0,... ? 00. 18 Ar 日arrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: 0.2 mol of KOH is added to 1.0 L of a 0.5 M HF solution. bases: Х other: ☐ acids: 0.10 mol of HI is added to 1.0 L of a solution that is 1.4M in both HF and NaF. bases: other: ☐ 0,0,... ด ? 18 Ararrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. acids: ☐ 1.8 mol of HCl is added to 1.0 L of a 1.0M NH3 bases: ☐ solution. other: ☐ 0.18 mol of HNO3 is added to 1.0 L of a solution that is 1.4M in both NH3 and NH₁Br. acids: bases: ☐ other: ☐ 0,0,... ? 000 18 Ar B 1arrow_forward
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NH3 (g) = N2 (g) +3H₂ —N2 (g) AGº = 34. kJ Now suppose a reaction vessel is filled with 4.19 atm of ammonia (NH3) and 9.94 atm of nitrogen (N2) at 378. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of NH 3 tend to rise or fall? ☐ x10 fall Х Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH 3 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no atm 00. 18 Ar 무ㅎ ?arrow_forwardIdentifying the major species in weak acid or weak base equilibria The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. 2.2 mol of NaOH is added to 1.0 L of a 1.4M HF solution. acids: П bases: Х other: ☐ ப acids: 0.51 mol of KOH is added to 1.0 L of a solution that is bases: 1.3M in both HF and NaF. other: ☐ 00. 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forward
- Homework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forwardIdentifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forward
- Highlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward€ + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forwardDraw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





