
Concept explainers
(a)
Interpretation:
It is to be determined whether and how[DK1] the given ether can be produced from a
Concept introduction:
The Williamson ether synthesis is the most convenient method for an ether synthesis. In this synthesis, an
Answer to Problem 10.16P
The given ether can be produced successfully from Williamson ether synthesis as below:
Explanation of Solution
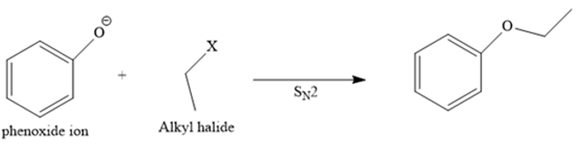
The structure of the given ether is
In this ether, one of the R groups is a phenyl ring, and the other is an ethyl group.
So, there are two routes to produce the desired ether by Williamson ether synthesis. Route one is discussed below.
Route I:
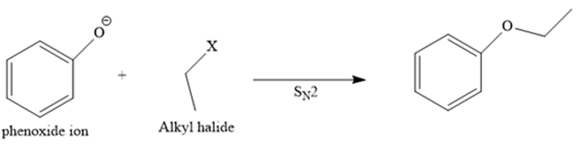
This is a feasible synthesis because the phenoxide ion is a good nucleophile, and the halide group attached on the primary carbon atom (primary alkyl halide) is a good substrate for a
The second possible route is discussed below.
Route II:
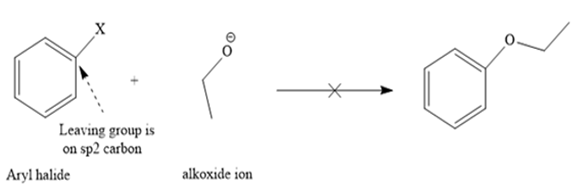
In this method, the halide group is on sp2 hybridized carbon, which is not acceptable for an
As Williamson ether synthesis is an
(b)
Interpretation:
It is to be determined whether and how[DK2] the given ether can be produced from a Williamson ether synthesis. And if there are two feasible syntheses for the given ether, it is to be determined which one is more preferable.
Concept introduction:
The Williamson ether synthesis is the most convenient method for an ether synthesis. In this synthesis, an alkyl halide
Answer to Problem 10.16P
The given ether cannot be synthesized by Williamson ether synthesis.
Explanation of Solution
The structure of the given ether is
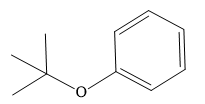
In this ether, one of the R groups is a phenyl ring, and the other is a tertiary butyl group. Those two groups would be the potential alkyl halides for a Williamson ether synthesis reaction. Route I is shown below:
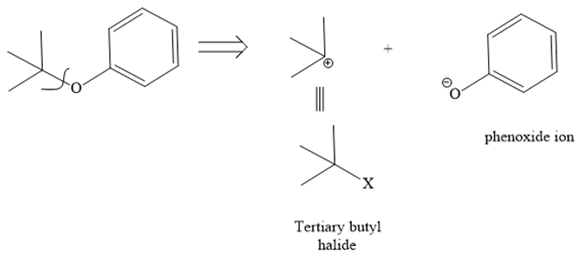
The retrosynthesis suggests that the given ether can be synthesized from a tertiary butyl halide as a substrate and a phenoxide ion as a nucleophile. But the alkyl halide has a leaving group on the tertiary carbon, so it will not follow an
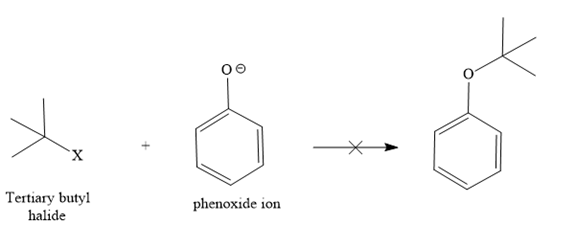
Instead, it shows an E1 reaction with the phenoxide ion because the phenoxide ion acts as a base instead of the nucleophile due to bulkiness.
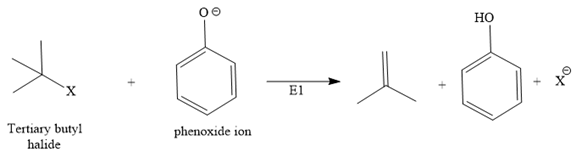
The second route is not acceptable because the positive charge comes on [DK4] the carbon atom of a phenyl ring, which is already electron-rich and sp2, which is not good for the
Route II:
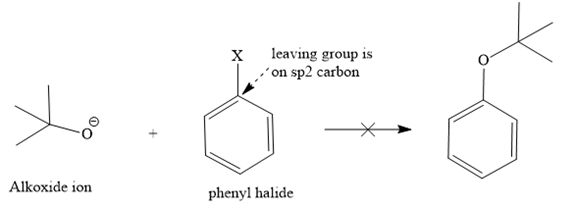
Since both routes do not give the desired ether as a product via
As Williamson ether synthesis is an
(c)
Interpretation:
It is to be determined whether and how[DK5] the given ether can be produced from a Williamson ether synthesis. And if there are two feasible syntheses for the given ether, it is to be determined which one is more preferable.
Concept introduction:
The Williamson ether synthesis is the most convenient method for an ether synthesis. In this synthesis, an alkyl halide
Answer to Problem 10.16P
The given ether can be successfully produced from a Williamson ether synthesis via two routes as below:
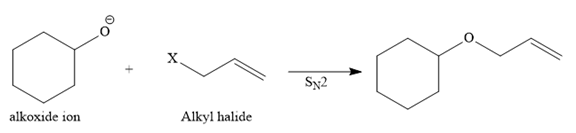
Route I:
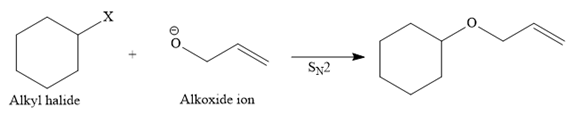
Route II:
The first route is more preferable as it makes use of a primary alkyl halide as a substrate.
Explanation of Solution
The given ether is
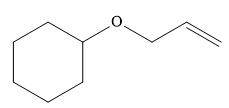
One R group in the given ether is cyclohexane while the other is an allyl group. Both of these R groups can be potentially used as substrates in the Williamson ether synthesis. Route I is shown below:
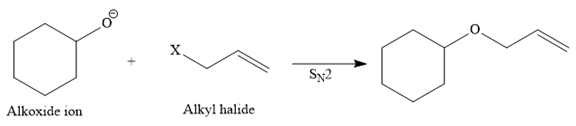
In this route, the leaving group (halogen atom, X) is on the primary carbon, and an alkoxide ion is also a good nucleophile, so the reaction can proceed through
The other route for the synthesis of the given ether is shown below:
Route II:

In this route, the leaving group (halogen atom, X) is on the secondary carbon, and an alkoxide ion is also a good nucleophile, so the reaction can proceed through
Note that both routes are feasible for the given ether synthesis, but the substrate of both routes is different. In the first route, the substrate (alkyl halide) has a leaving group on primary carbon while in the second route it is on the secondary carbon. Since an
As Williamson ether synthesis is an
Want to see more full solutions like this?
Chapter 10 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Don't used hand raitingarrow_forwardGramicidin A can adopt more than one structure; NMR spectroscopy has revealed an “end-to-end” dimer form, and x-ray crystallography has revealed an “anti-parallel double- helical” form. Briefly outline and describe an experimentalapproach/strategy to investigate WHICH configuration (“end-to-end dimer” vs “anti-paralleldouble helical”) gramicidin adopts in an actual lipid bilayer.arrow_forwardDon't used hand raitingarrow_forward
- CHEM2323 Problem 2-24 Tt O e: ל Predict the product(s) of the following acid/base reactions. Draw curved arrows to show the formation and breaking of bonds. If the bonds needed are not drawn out, you should redraw them. + BF3 (a) (b) HI + (c) OH -BF Problem 2-25 Use curved arrows and a proton (H+) to draw the protonated form of the following Lewis bases. Before starting, add all missing lone pairs. (a) (b) :0: (c) N 1 CHEM2323 PS CH02 Name:arrow_forwardCHEM2323 Problem 2-26 Tt O PS CH02 Name: Use the curved-arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right. (Draw all lone pairs first) (a) NH2 NH2 + (b) Problem 2-27 Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each of the following double bonds will react with H-Cl and draw the resulting carbocation. (a) H2C=CH2 (b) (c) Problem 2-28 Identify the most electronegative element in each of the following molecules: (a) CH2FCI F Problem 2-29 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li F 0 0 Use the electronegativity table in Figure 2.3 to predict which bond in the following pairs is more polar and indicate the direction of bond polarity for each compound. (a) H3C-Cl or Cl-CI (b) H3C-H or H-CI (c) HO-CH3 or (CH3)3Si-CH3 (d) H3C-Li or Li-OHarrow_forwardDon't used hand raitingarrow_forward
- Don't used hand raitingarrow_forwardat 32.0 °C? What is the osmotic pressure (in atm) of a 1.46 M aqueous solution of urea [(NH2), CO] at 3 Round your answer to 3 significant digits.arrow_forwardReagan is doing an atomic absorption experiment that requires a set of zinc standards in the 0.4-1.6 ppm range. A 1000 ppm Zn solution was prepared by dissolving the necessary amount of solid Zn(NO3)2 in water. The standards can be prepared by diluting the 1000 ppm Zn solution. Table 1 shows one possible set of serial dilutions (stepwise dilution of a solution) that Reagan could perform to make the necessary standards. Solution A was prepared by diluting 5.00 ml of the 1000 ppm Zn standard to 50.00 ml. Solutions C-E are called "calibration standards" because they will be used to calibrate the atomic absorption spectrometer. a. Compare the solution concentrations expressed as ppm Zn and ppm Zn(NO3)2. Compare the concentrations expressed as M Zn and M Zn(NO3)2 - Which units allow easy conversion between chemical species (e.g. Zn and Zn(NO3)2)? - Which units express concentrations in numbers with easily expressed magnitudes? - Suppose you have an analyte for which you don't know the molar…arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
