
Interpretation:
Possible monochlorinated products has to be given and the products has to be named using
Concept Introduction:
Halogenation reaction is the one where atom or atoms of halogens get substituted in a carbon chain. Halogenation is a type of substitution reaction.
In
IUPAC rules for naming alkanes:
There are about five rules that has to be followed for naming an alkane and they are,
- The longest continuous carbon chain in the compound has to be identified. This is known as parent compound. From this the parent name is obtained. Suffix “–ane” (for alkane) is added at the end of the prefix which gives information about the number of carbon atoms.
- Numbering has to be done so that the lowest number is given to the first group that is encountered in the parent chain.
- Naming and numbering has to be given for each atom or group that is attached to the parent chain. Numbering has to be done in a way that substituents get the least numbering.
- If the same substitution is present in the parent chain more than once, a separate prefix is added which tells about the number of times the substituent occurs. Prefixes used are di-, tri-, tetra-, penta- etc.
- Name of the substituents has to be placed in an alphabetical order before the parent compound name.
Explanation of Solution
Structure of given alkane is,
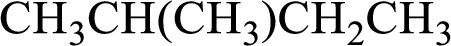
Monochlorination of
IUPAC name for
In the given compound, the longest carbon chain is found to contain four carbon atoms. Therefore, the parent alkane name is butane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
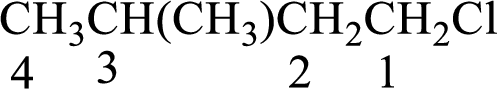
The substituent present in the given compound are a chlorine atom and a methyl group. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
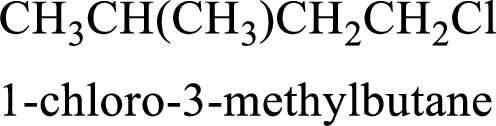
Parent chain is butane and the substituent present is 1-chloro-3-methyl. Hence, the IUPAC name is given as 1-chloro-3-methylbutane.
IUPAC name for
In the given compound, the longest carbon chain is found to contain four carbon atoms. Therefore, the parent alkane name is butane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
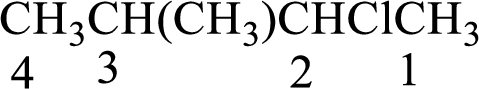
The substituent present in the given compound are a chlorine atom and a methyl group. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
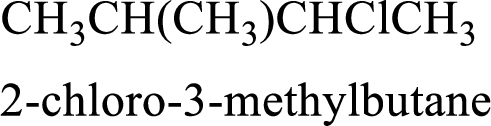
Parent chain is butane and the substituent present is 2-chloro-3-methyl. Hence, the IUPAC name is given as 2-chloro-3-methylbutane.
IUPAC name for
In the given compound, the longest carbon chain is found to contain four carbon atoms. Therefore, the parent alkane name is butane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
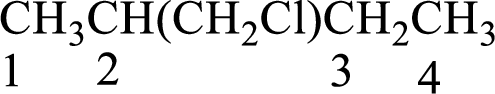
The substituent present in the given compound are a chloromethyl group. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
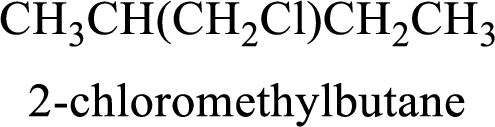
Parent chain is butane and the substituent present is 2-chloromethyl. Hence, the IUPAC name is given as 2-chloromethylbutane.
IUPAC name for
In the given compound, the longest carbon chain is found to contain four carbon atoms. Therefore, the parent alkane name is butane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
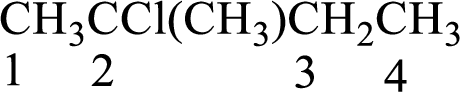
The substituent present in the given compound are a chlorine atom and a methyl group. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
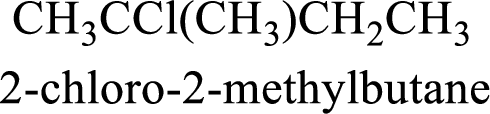
Parent chain is butane and the substituent present is 2-chloro-2-methyl. Hence, the IUPAC name is given as 2-chloro-2-methylbutane.
IUPAC name for
In the given compound, the longest carbon chain is found to contain four carbon atoms. Therefore, the parent alkane name is butane.
Numbering of carbon atoms has to be done in a way that the substituents present in the longest carbon chain get the least numbering.
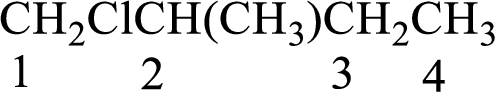
The substituent present in the given compound are a chlorine atom and a methyl group. Number has to be added before the substituent indicating the carbon in which it is attached. Therefore, IUPAC name is obtained as,
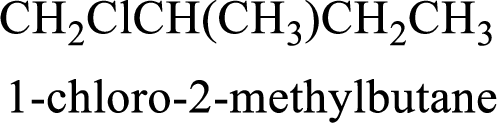
Parent chain is butane and the substituent present is 1-chloro-2-methyl. Hence, the IUPAC name is given as 1-chloro-2-methylbutane.
Want to see more full solutions like this?
Chapter 10 Solutions
Connect 1-Semester Access Card for General, Organic, and Biochemistry
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





