
Study Guide/solutions Manual For Organic Chemistry
6th Edition
ISBN: 9781260475678
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 43P
Assign formal charges to each
a. 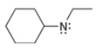 b.
b. 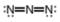 c.
c. 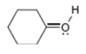 d.
d. 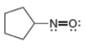
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the products of the reaction shown below. Use wedge and dash bonds
to indicate stereochemistry. Ignore inorganic byproducts.
OSO4 (cat)
(CH3)3COOH
Select to Draw
ઘ
Calculate the reaction rate for selenious acid, H2SeO3, if 0.1150 M I-1 decreases to 0.0770 M in 12.0 minutes.
H2SeO3(aq) + 6I-1(aq) + 4H+1(aq) ⟶ Se(s) + 2I3-1(aq) + 3H2O(l)
Problem 5-31
Which of the following objects are chiral?
(a) A basketball
(d) A golf club
(b) A fork
(c) A wine glass
(e) A spiral staircase
(f) A snowflake
Problem 5-32
Which of the following compounds are chiral? Draw them, and label the chirality centers.
(a) 2,4-Dimethylheptane
(b) 5-Ethyl-3,3-dimethylheptane
(c) cis-1,4-Dichlorocyclohexane
Problem 5-33
Draw chiral molecules that meet the following descriptions:
(a) A chloroalkane, C5H11Cl
(c) An alkene, C6H12
(b) An alcohol, C6H140
(d) An alkane, C8H18
Problem 5-36
Erythronolide B is the biological precursor of
erythromycin, a broad-spectrum antibiotic. How
H3C
CH3
many chirality centers does erythronolide B have?
OH
Identify them.
H3C
-CH3
OH
Erythronolide B
H3C.
H3C.
OH
OH
CH3
Chapter 1 Solutions
Study Guide/solutions Manual For Organic Chemistry
Ch. 1.1 - While the most common isotope of nitrogen has a...Ch. 1.2 - Label each bond in the following compounds as...Ch. 1.3 - Draw a valid Lewis structure for each species. a....Ch. 1.3 - Prob. 9PCh. 1.4 - Draw Lewis structures for each molecular formula....Ch. 1.6 - Classify each pair of compounds as isomers or...Ch. 1.6 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Prob. 14PCh. 1.6 - Prob. 16P
Ch. 1.6 - Prob. 17PCh. 1.7 - Prob. 18PCh. 1.7 - Prob. 19PCh. 1.7 - Using the principles of VSEPR theory, you can...Ch. 1.8 - Convert each condensed formula to a Lewis...Ch. 1.8 - Prob. 22PCh. 1.8 - Prob. 23PCh. 1.8 - Convert each skeletal structure to a complete...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Assign formal charges to each and atom in the...Ch. 1 - Prob. 44PCh. 1 - Prob. 46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Consider compounds A-D, which contain both a...Ch. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - 1.61 Convert each molecule into a skeletal...Ch. 1 - Prob. 65PCh. 1 - Predict the hybridization and geometry around each...Ch. 1 - Prob. 68PCh. 1 - Ketene, , is an unusual organic molecule that has...Ch. 1 - Rank the following bonds in order of increasing...Ch. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Prob. 74PCh. 1 - Anacin is an over-the-counter pain reliever that...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 83PCh. 1 - Prob. 84PCh. 1 - Prob. 85PCh. 1 - Prob. 86PCh. 1 - Prob. 87PCh. 1 - Prob. 88P
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
The validity of a scientific law.
Physical Universe
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Give the IUPAC name for each compound.
Organic Chemistry
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Problem 5-48 Assign R or S configurations to the chirality centers in ascorbic acid (vitamin C). OH H OH HO CH2OH Ascorbic acid O H Problem 5-49 Assign R or S stereochemistry to the chirality centers in the following Newman projections: H Cl H CH3 H3C. OH H3C (a) H H H3C (b) CH3 H Problem 5-52 Draw the meso form of each of the following molecules, and indicate the plane of symmetry in each: OH OH (a) CH3CHCH2CH2CHCH3 CH3 H3C. -OH (c) H3C CH3 (b) Problem 5-66 Assign R or S configurations to the chiral centers in cephalexin, trade-named Keflex, the most widely prescribed antibiotic in the United States. H2N H IHH S Cephalexin N. CH3 CO₂Harrow_forwardSteps and explanationn please.arrow_forwardSteps and explanationn please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY