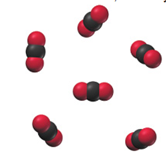Chemistry: Structure and Properties (2nd Edition)
2nd Edition
ISBN: 9780134293936
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 41E
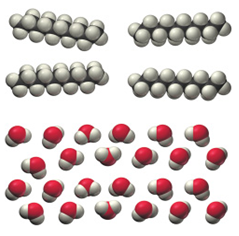
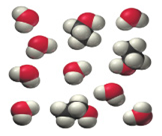
Determine whether each molecular diagram represents a pure substance or a mixture. If it represents a pure substance, classify the substance as an element or a compound. If it represents a mixture, classify the mixture as homogeneous or heterogeneous.

Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Don't used Ai solution
Don't used Ai solution
The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.
Chapter 1 Solutions
Chemistry: Structure and Properties (2nd Edition)
Ch. 1 - Explain this statement in your own words and give...Ch. 1 - Explain the main goal of chemistry.Ch. 1 - What are two different ways to classify matter?Ch. 1 - How do solids, liquids, and gases differ?Ch. 1 - Explain the difference between a pure substance...Ch. 1 - Explain the difference between an element and a...Ch. 1 - Explain the difference between a homogeneous and a...Ch. 1 - Describe the scientific approach to knowledge. How...Ch. 1 - Prob. 9ECh. 1 - What observations did Antoine Lavoisier make? What...
Ch. 1 - What theory did John Dalton formulate?Ch. 1 - What is wrong with the expression, “That is just a...Ch. 1 - Summarize the history of the atomic idea. How was...Ch. 1 - Prob. 14ECh. 1 - State and explain the law of definite proportions.Ch. 1 - State and explain the law of multiple proportions....Ch. 1 - What are the main ideas in Dalton’s atomic theory?...Ch. 1 - How and by whom was the electron discovered? What...Ch. 1 - Explain Millikan’s oil drop experiment and how it...Ch. 1 - Prob. 20ECh. 1 - Describe Rutherford’s gold foil experiment. How...Ch. 1 - Describe Rutherford’s nuclear model of the atom....Ch. 1 - If matter is mostly empty space, as suggested by...Ch. 1 - List the three subatomic particles that compose...Ch. 1 - What defines an element?Ch. 1 - Explain the difference between Z (the atomic...Ch. 1 - Where do elements get their names?Ch. 1 - What are isotopes? What is percent natural...Ch. 1 - Describe the two different notations used to...Ch. 1 - Prob. 30ECh. 1 - Prob. 31ECh. 1 - Explain how a mass spectrometer works. What kind...Ch. 1 - What is a mole? How is the mole concept useful in...Ch. 1 - Prob. 34ECh. 1 - Each shape represents a type of particle (such as...Ch. 1 - Using triangles to represent one type of atom and...Ch. 1 - Classify each substance as a pure substance or a...Ch. 1 - Classify each substance as a pure substance or a...Ch. 1 - Prob. 39ECh. 1 - Complete the table. Substance Pure or mixture Type...Ch. 1 - Determine whether each molecular diagram...Ch. 1 - Determine whether each molecular diagram...Ch. 1 - Classify each statement as an observation, a law,...Ch. 1 - Classify each statement as an observation, a law,...Ch. 1 - A chemist decomposes several samples of carbon...Ch. 1 - When astronomers observe distant galaxies, they...Ch. 1 - Prob. 47ECh. 1 - An automobile gasoline tank holds 21 kg of...Ch. 1 - Two samples of carbon tetrachloride are decomposed...Ch. 1 - Two samples of sodium chloride are decomposed into...Ch. 1 - The mass ratio of sodium to fluorine in sodium...Ch. 1 - Upon decomposition, one sample of magnesium...Ch. 1 - Two different compounds containing osmium and...Ch. 1 - Palladium forms three different compounds with...Ch. 1 - Prob. 55ECh. 1 - Sulfur and fluorine form several different...Ch. 1 - Which statements are consistent with Dalton’s...Ch. 1 - Which statements are inconsistent with Dalton’s...Ch. 1 - Which statements are consistent with Rutherford’s...Ch. 1 - Which statements are inconsistent with...Ch. 1 - A chemist in an imaginary universe, where...Ch. 1 - Imagine a unit of charge called the zorg. A...Ch. 1 - Which statements about subatomic particles are...Ch. 1 - Which statements about subatomic particles are...Ch. 1 - Write isotopic symbols in the form XA (e g., C-13)...Ch. 1 - Write isotopic symbols in the form ZAX for each...Ch. 1 - Determine the number of protons and the number of...Ch. 1 - Determine the number of protons and the number of...Ch. 1 - The amount of carbon-14 in ancient artifacts and...Ch. 1 - Uranium-235 is used in nuclear fission. Determine...Ch. 1 - Determine the number of protons and the number of...Ch. 1 - Determine the number of protons and the number of...Ch. 1 - Gallium has two naturally occurring isotopes with...Ch. 1 - Magnesium has three naturally occurring isotopes...Ch. 1 - The atomic mass of fluorine is 18.998 amu, and its...Ch. 1 - The atomic mass of copper is 63.546 amu. Do any...Ch. 1 - An element has two naturally occurring isotopes....Ch. 1 - An element has four naturally occuring isotopes...Ch. 1 - Bromine has two naturally occurring isotopes...Ch. 1 - Silicon has three naturally occurring isotopes...Ch. 1 - Use the mass spectrum of europium shown here to...Ch. 1 - Use the mass spectrum of rubidium shown here to...Ch. 1 - How many sulfur atoms are there in 5.52 mol of...Ch. 1 - How many moles of aluminum do 3.71024 aluminum...Ch. 1 - What is the amount, in moles, of each elemental...Ch. 1 - What is the mass, in grams, of each elemental...Ch. 1 - How many silver atoms are there in 3.78 g of...Ch. 1 - What is the mass of 4.91 x 1021 platinum atoms?Ch. 1 - Calculate the number of atoms in each sample. 5.18...Ch. 1 - Calculate the number of atoms in each sample...Ch. 1 - Calculate the mass in grams, of each sample. 1.1 x...Ch. 1 - Calculate the mass, in kg, of each sample. 7.55 x...Ch. 1 - How many carbon atoms are there in a diamond (pure...Ch. 1 - How many helium atoms are there in a helium blimp...Ch. 1 - Calculate the average mass, in grams, of one...Ch. 1 - Using scanning tunneling microscopy, scientists at...Ch. 1 - A 7.83-g sample of HCN contains 0.290 g of H and...Ch. 1 - The ratio of sulfur to oxygen by mass in SO2 is...Ch. 1 - Use the mass spectrum of lead shown here to...Ch. 1 - Use the mass spectrum of mercury shown here to...Ch. 1 - Nuclei with the same number of neutrons but...Ch. 1 - Fill in the blanks to complete the table. Symbol z...Ch. 1 - A penny has a thickness of approximately 1.0 mm....Ch. 1 - Consider the stack of pennies in Problem 103. How...Ch. 1 - A pure copper sphere has a radius of 0.935 in. How...Ch. 1 - A pure titanium cube has an edge length of 2.78...Ch. 1 - A 67.2-g sample of a gold and palladium alloy...Ch. 1 - Common brass is a copper and zinc alloy containing...Ch. 1 - The U.S. Environmental Protection Agency (EPA)...Ch. 1 - Pure gold is usually too soft for jewelry, so it...Ch. 1 - Silver is composed of two naturally occurring...Ch. 1 - To the right is a representation of 50 atoms of a...Ch. 1 - The ratio of oxygen to nitrogen by mass in NO2 is...Ch. 1 - Naturally occurring cobalt consists of only one...Ch. 1 - A 7.36-g sample of copper is contaminated with an...Ch. 1 - The ratio of the mass of O to the mass of N in...Ch. 1 - Naturally occurring magnesium has an atomic mass...Ch. 1 - In Section 1.10 O, it was stated that 1 mol of...Ch. 1 - Use the concepts in this chapter to obtain an...Ch. 1 - A volatile liquid (one that readily evaporates) is...Ch. 1 - The diagram to the right represents solid carbon...Ch. 1 - Use triangles to represent atoms of element A and...Ch. 1 - Identify each statement as being most like an...Ch. 1 - The mole is defined as the amount of a substance...Ch. 1 - Prob. 125ECh. 1 - Using white and black circles to represent...Ch. 1 - In a naturally occurring sample, 19.8% of boron...Ch. 1 - In complete sentences, describe the similarities...Ch. 1 - Calculate the mass in grams of one mole of each of...Ch. 1 - The U.S. Environmental Protection Agency (U.S....Ch. 1 - This image represents a particulate view of a...Ch. 1 - A chemist mixes sodium with water and witnesses a...Ch. 1 - Two samples of a compound containing elements A...Ch. 1 - A compound containing only carbon and hydrogen has...Ch. 1 - Which concept was demostrated by Rutherford’s gold...Ch. 1 - A student re-creates Millikan’s oil drop...Ch. 1 - Prob. 7SAQCh. 1 - An isotope of an element contains 82 protons and...Ch. 1 - How many electrons are in the Cr3+ ion? 24...Ch. 1 - A naturally occurring sample of an element...Ch. 1 - Copper has an atomic mass of 63.55 amu and two...Ch. 1 - Which sample contains the greatest number of...Ch. 1 - A solid copper cube contains 4.31023 atoms. What...Ch. 1 - Determine the number of atoms in 1.85 mL of...Ch. 1 - A 20.0-g sample of an element contains 4.951023...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Electronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardCalculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forward
- General formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward
- (please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forwardIn the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forward
- The degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forwardIn natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forwardMost ceramic materials have low thermal conductivities because:(a) Electron mobility is strongly restricted due to their strong ionic-covalent bonding.(b) False, in general they are excellent thermal conductors (they are used in ovens).(c) Electron mobility is dependent on T and therefore they are poor conductors at high temperatures.(d) Electron mobility is very restricted by secondary bonds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY