
Concept explainers
(a)
Interpretation:
All the constitutional isomers that have the molecular formula
Concept Introduction:
- Isomers are compounds having same molecular formula but has different arrangements of atoms
- Constitutional isomers have same molecular formula but differ in the way their atoms are connected.
Steps to draw the constitutional isomers
- Valency of each atom that appears in the molecular formula has to be known.
- Atom with highest valency has to be connected first and the monovalent atom has to be placed at the periphery.
(a)
Explanation of Solution
Given molecular formula
Carbon is tetravalent and hydrogen is monovalent.
Since Carbon is tetravalent, it has to be connected first as given below,
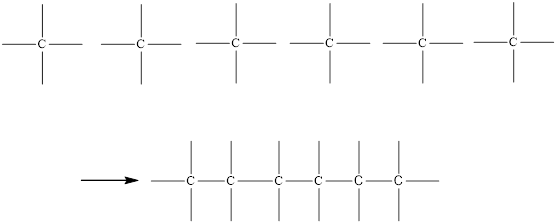
The atom that has more than one bond is carbon and it should be drawn in the center of the compound.
On placing the hydrogen atom at the periphery, the isomers will be
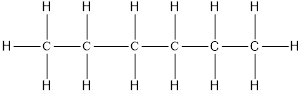
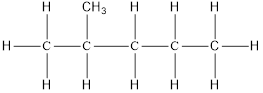
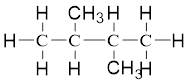
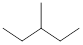 and
and 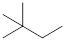
(b)
Interpretation:
All the constitutional isomers that have the molecular formula
Concept Introduction:
- Isomers are compounds having same molecular formula but has different arrangements of atoms
- Constitutional isomers have same molecular formula but differ in the way their atoms are connected.
Steps to draw the constitutional isomers
- Valency of each atom that appears in the molecular formula has to be known.
- Atom with highest valency has to be connected first and the monovalent atom has to be placed at the periphery.
(b)
Explanation of Solution
Given molecular formula
Carbon is tetravalent, chlorine and hydrogen is monovalent.
Since Carbon is tetravalent, it has to be connected first as given below,

The atom that has more than one bond is carbon and it should be drawn in the center of the compound.
Here chlorine can be placed only at positions, i.e. connected to any one of the carbon.
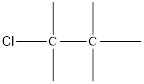
On placing the hydrogen atom at the periphery, the isomer will be
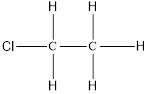
(c)
Interpretation:
All the constitutional isomers that have the molecular formula
Concept Introduction:
- Isomers are compounds having same molecular formula but has different arrangements of atoms
- Constitutional isomers have same molecular formula but differ in the way their atoms are connected.
Steps to draw the constitutional isomers
- Valency of each atom that appears in the molecular formula has to be known.
- Atom with highest valency has to be connected first and the monovalent atom has to be placed at the periphery.
(c)
Explanation of Solution
Given molecular formula
Carbon is tetravalent, chlorine and hydrogen is monovalent.
Since Carbon is tetravalent, it has to be connected first as given below,

The atom that has more than one bond is carbon and it should be drawn in the center of the compound.
Here chlorine can be placed at two positions,
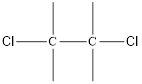 and
and 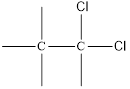
On placing the hydrogen atom at the periphery, the isomer will be
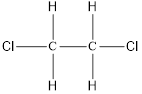 and
and 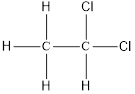
(c)
Interpretation:
All the constitutional isomers that have the molecular formula
Concept Introduction:
- Isomers are compounds having same molecular formula but has different arrangements of atoms
- Constitutional isomers have same molecular formula but differ in the way their atoms are connected.
Steps to draw the constitutional isomers
- Valency of each atom that appears in the molecular formula has to be known.
- Atom with highest valency has to be connected first and the monovalent atom has to be placed at the periphery.
(c)
Explanation of Solution
Given molecular formula
Carbon is tetravalent, chlorine and hydrogen is monovalent.
Since Carbon is tetravalent, it has to be connected first as given below,

The atom that has more than one bond is carbon and it should be drawn in the center of the compound.
Here chlorine can be placed at two positions.
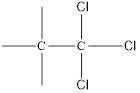 and
and 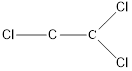
On placing the hydrogen atom at the periphery, the isomer will be
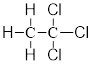 and
and 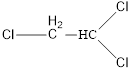
Want to see more full solutions like this?
Chapter 1 Solutions
ORGANIC CHEMISTRY-STD.WILEY PLUS CARD
- For each reaction below, decide if the first stable organic product that forms in solution will create a new C-C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 CI MgCl ? Will the first product that forms in this reaction create a new CC bond? Yes No MgBr ? Will the first product that forms in this reaction create a new CC bond? Yes No G टेarrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new CC bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. དྲ。 ✗MgBr ? O CI Will the first product that forms in this reaction create a new C-C bond? Yes No • ? Will the first product that forms in this reaction create a new CC bond? Yes No × : ☐ Xarrow_forwardPredict the major products of this organic reaction: OH NaBH4 H ? CH3OH Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. ☐ : Sarrow_forward
- Predict the major products of this organic reaction: 1. LIAIHA 2. H₂O ? Note: be sure you use dash and wedge bonds when necessary, for example to distinguish between major products with different stereochemistry. Click and drag to start drawing a structure. X : ☐arrow_forwardFor each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 tu ? ? OH Will the first product that forms in this reaction create a new CC bond? Yes No Will the first product that forms in this reaction create a new CC bond? Yes No C $ ©arrow_forwardAs the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C-C bond as its major product: 1. MgCl ? 2. H₂O* If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new CC bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new CC bond. G marrow_forward
- Including activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M NH4 Ksp Hg2Br2 = 5.6×10-23.arrow_forwardgive example for the following(by equation) a. Converting a water insoluble compound to a soluble one. b. Diazotization reaction form diazonium salt c. coupling reaction of a diazonium salt d. indacator properties of MO e. Diazotization ( diazonium salt of bromobenzene)arrow_forward2-Propanone and ethyllithium are mixed and subsequently acid hydrolyzed. Draw and name the structures of the products.arrow_forward
- (Methanesulfinyl)methane is reacted with NaH, and then with acetophenone. Draw and name the structures of the products.arrow_forward3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.arrow_forwardWhat is the reason of the following(use equations if possible) a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5 d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt e.) Cbvcarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





