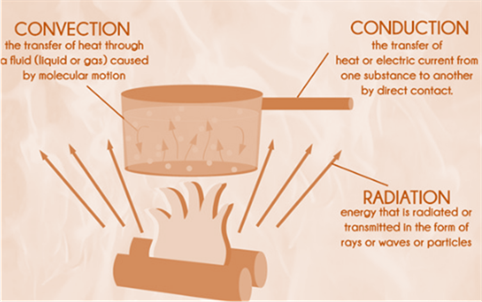
Problem 1CP: How does the science of heat transfer differ from the science of thermodynamics? Problem 2CP: What is the driving force for (a) heat transfer, (b) electric current flow; and (c) fluid flow? Problem 3CP: How do rating problems in heat transfer differ from the sizing problems? Problem 4CP: What is the difference between the analytical and experimental approaches to heat transfer? Discuss... Problem 5CP: What is the importance of modeling in engineering? How are the mathematical models for engineering... Problem 6CP: When modeling an engineering process, how is the right choice made between a simple but crude and a... Problem 7CP: On a hot summer day, a student turns his fan on when he leaves his room in the morning. When he... Problem 8CP: Consider two identical rooms, one with a refrigerator in it and the other without one. If all the... Problem 9CP Problem 10CP Problem 11CP Problem 12CP: An ideal gas is heated from 50C to 80C (a) at constant volume and (b) at constant pressure. For... Problem 13CP: What is heat flux? How is it related to the heat transfer rate? Problem 14CP: What are the mechanisms of energy transfer to a closed system? How is heat transfer distinguished... Problem 15EP: A logic chip used in a computer dissipates 3 W of power in an environment at 120F and has a heat... Problem 16P: Consider a 150-W incandescent lamp. The filament of the lamp is 5 cm long and has a diameter of 0.5... Problem 17P: A 15-cm-diameter aluminum ball is to be heated form 80C and to an average temperature of 200C. and... Problem 18EP: A 60-gallon water heated is initially filled with water at 50F. Determine how much energy (in Btu)... Problem 19P Problem 20P Problem 21P Problem 22P Problem 23P Problem 24P Problem 25P Problem 26P Problem 27P: A 5-m6-m8-m room is to be heated by an electrical resistance heater placed in a short duct in the... Problem 28P Problem 29EP: Air enters the duct of an air-conditioning system at 15 psia and 47F at a volume flow rate of 450ft3... Problem 30P Problem 31CP: Define thermal conductivity, and explain its significance in heat transfer. Problem 32CP: Which is a better heat conductor, diamond or silver? Problem 33CP: How do the thermal conductivity of gases and liquids vary with temperature? Problem 34CP: Why is the thermal conductivity of superinsulation order of magnitude lower than the thermal... Problem 35CP: Why do we characterize the heat conduction ability of insulators in terms of their apparent thermal... Problem 36CP: What are the mechanisms of heat transfer? How are they distinguished from each other? Problem 37CP: Write down the expression for the physical laws that govern each mode of heat transfer, and identify... Problem 38CP: How does heat conduction differ from convection? Problem 39CP: Does any of the energy of the sun reach the earth by conduction or convection? Problem 40CP: How does forced convection differ from natural convection? Problem 41CP: What is the physical mechanism of heat conduction in a solid, a liquid, and a gas? Problem 42CP: Consider heat transfer a windowless wall of house on a winter day. Discuss the parameter that effect... Problem 43CP: Consider heat loss through two walls of house on a winter night. The Walls are identical except that... Problem 44CP: Consider two houses that are identical except that the walls are built using bricks in one house and... Problem 45CP: Consider two walls of a house that are identical except that one is made of 10-cm-thick wood while... Problem 46CP: Define emissivity and absorptivity. What is Kirchhoffs law of radiation? Problem 47CP: What is a blackbody? How do real bodies differ from blackbodies? Problem 48P: A wood slab with a thickness 0.05 m is subjected to a heat flux of 40 W/m2 . The left and right... Problem 49P Problem 50EP Problem 51P: The inner and outer surfaces of a 0.5-cm thick 2-m2-m glass in winter are 10C and 3C, respectively.... Problem 52P Problem 53P Problem 54EP: The north wall of an electrically heated home is 20ft long, 10ft high, and 1ft thick and is made of... Problem 55P Problem 56P Problem 57P Problem 58P: A concreate wall a surface area of 20 m2 and a thickness of 0.30 m separates conditioned room air... Problem 59P Problem 60P Problem 61P Problem 62EP Problem 63P: Air at 20C with a convection heat transfer coefficient of 20 W/m2 .K blows over a pond. The surface... Problem 64P Problem 65P Problem 66P Problem 67P Problem 68P Problem 69P Problem 70P Problem 71P Problem 72EP Problem 73P Problem 74P Problem 75P Problem 76P Problem 77EP: Using the conversion factors between W and Btu/h, m and ft, and K and R, express the... Problem 78P: The outer surface of a spacecraft in space has an emissivity of 0.8 and a solar absorptivity of 0.3.... Problem 79P: Consider a person whose expose surface are is 1.7 m2 , emissivity is 0.5, and surface temperature is... Problem 80P Problem 81P: Two surfaces, one highly polished and the other heavily oxidized, are found to be emitting the same... Problem 82P: A spherical interplanetary probe with a diameter of 2 m is sent out into the solar system. The probe... Problem 83P Problem 84CP: Can all three modes of heat transfer occur simultaneously (in parallel) in a medium? Problem 85CP: Can a medium involve (a) conduction and convection, (b) conduction and radiation, or (c) convection... Problem 86CP: The deep human body temperature of a healthy person remains constant at 37oC while the temperature... Problem 87CP: We often turn the fan on in summer to help us cool. Explain how a fan makes us feel cooler in the... Problem 88P Problem 89P Problem 90P Problem 91P: An electronic package with a surface area of 1 m2 placed in an orbiting space station is exposed to... Problem 92P: Consider steady heat transfer between two large parallel plates at constant temperatures of T1=290K... Problem 93P Problem 94P Problem 95EP: A 2-in-diameter spherical ball whose surface is maintained at a temperature of 170oF is suspended in... Problem 96P Problem 97P Problem 98P: A 3-m-internal-diameter spherical tank made of 1-cm-thick stainless steel is use to store iced water... Problem 99P Problem 100P: Solar radiation is incident on a 5-m2 solar absorber plate surface at a rate of 800 W/m2.... Problem 101P Problem 102P Problem 103EP Problem 104P: An AISI 304 stainless steel sheet is going through an annealing process inside an electrically... Problem 105P Problem 106P Problem 107P Problem 108CP Problem 109P Problem 110P Problem 111P Problem 112P Problem 113CP Problem 114CP: Why is the metabolic rate of women, in general, lower than that of men? What is the effect of... Problem 115CP: What is asymmetric thermal radiation How does it cause thermal discomfort in the occupants of a... Problem 116CP: How do (a) draft and (b) cold floor surfaces cause discomfort for a rooms occupants? Problem 117CP Problem 118CP: Why is it necessary to ventilate buildings? What is the effect of ventilation on energy consumption... Problem 119P: Consider a house in Atlanta, Georgia, that is maintained at 22oC and has a total of 20 m2 of window... Problem 120P Problem 121P: A 4m5m6m and room is to be heated by one ton (1000 kg) of liquid water contained in a tank placed in... Problem 122P: Engine valves (cp=440J/kg.Kandp=7840kg/m3) are to be heated from 40oC to 800oC in 5 min in the heat... Problem 123P Problem 124P Problem 125P: A 0.3 -cm-thick, 12-cm-high, and 18-cm-long circuit board houses 80 closely spaced logic chips on... Problem 126P: A 40-cm-long, 800-W electric resistance heating element with dieter 0.5 cm and surface temperature... Problem 127P: It is well known that wind makes the cold air feel much colder as a result of the wind-chill effect... Problem 128P: An engine block with a surface area measured to be 0.95 m2 generates a power output of 50 kW with a... Problem 129P Problem 130P Problem 131P Problem 132P: Consider a person standing in a room maintained at 20oC at all times. The inner surfaces of the... Problem 133P Problem 134P Problem 135P Problem 136P Problem 137P Problem 138P Problem 139P Problem 140P Problem 141P Problem 142P Problem 143P: A 2-kW electric resistance heater submerged in 30-kg water is turned on and kept on for 10 min.... Problem 144P Problem 145P: A cold bottled drink (m=2.5kg,cp=4200J/kg.K) at 5oC is left on a table in a room. The average... Problem 146P Problem 147P: Air enters a 12-m-long, 7-cm-diameter pipe at 50oC at a rate of 0.06 kg/s. The air is cooled at an... Problem 148P Problem 149P: Steady heat conduction occurs through a 0.3-m-thick,9-m3-m composite wall at a rate of 1.2 kW. If... Problem 150P: Heat is lost through a brick wall (k=0.72W/m.K), which is 4 m long, 3 wide, and 25 cm thick at a... Problem 151P Problem 152P: A 40-cm-long, 0.4-cm-diameter electric resistance wire submerged in water is used to determine the... Problem 153P Problem 154P Problem 155P: Over 90 percent of the energy dissipated by an incandescent lightbulb is in the form of heat, not... Problem 156P: On a still, cleat night, the sky appears to be a blackbody with an equivalent temperature of 250 K.... Problem 157P Problem 158P Problem 159P: A persons head can be approximated as a 25-cm-diameter sphere at 35oC with an emissivity of 0.95.... Problem 160P: A person standing in a room loses heat to the air in the room by convection and to the surrounding... Problem 161P: Write an essay on how microwave ovens work, and explain how they cook much faster than conventional... Problem 162P: Using information form the utility bill for the coldest month last year, estimate the average rate... Problem 164P: It is well know that at the same outdoor air temperature a person is cooled at a faster rate under... format_list_bulleted

 Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning