
PRINCIPLES OF MODERN CHEMISTRY
8th Edition
ISBN: 9780357671009
Author: OXTOBY
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 18P
The natural abundances and 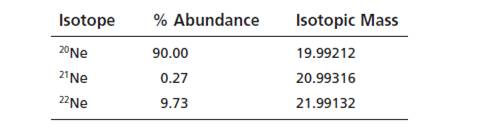
Calculate the atomic mass of naturally occurring neon.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the proper IUPAC name only for the following
compound. Dashes, commas, and spaces must be used
correctly, but do not use italics in Canvas.
The kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 M
What is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)
Chapter 1 Solutions
PRINCIPLES OF MODERN CHEMISTRY
Ch. 1 - Classify the following materials as substances or...Ch. 1 - Classify the following materials as substances or...Ch. 1 - A 17th-century chemist wrote of the “simple bodies...Ch. 1 - Since 1800, almost 200 sincere but erroneous...Ch. 1 - A sample of ascorbic acid (vitamin C) is...Ch. 1 - A sample of a compound synthesized and purified in...Ch. 1 - Nitrogen (N) and silicon (Si) form two binary...Ch. 1 - Iodine (I) and fluorine (F) form a series of...Ch. 1 - Vanadium (V) and oxygen (O) form a series of...Ch. 1 - Prob. 10P
Ch. 1 - Prob. 11PCh. 1 - Prob. 12PCh. 1 - Pure nitrogen dioxide (NO2) forms when dinitrogen...Ch. 1 - Gaseous methanol (CH3OH) reacts with oxygen (O2)...Ch. 1 - In J. J. Thompson’s experiment depicted in Figures...Ch. 1 - In the problem 15 above, what is vy , the...Ch. 1 - The natural abundances and isotopic masses of the...Ch. 1 - The natural abundances and isotopic masses of the...Ch. 1 - Prob. 19PCh. 1 - More than half of all the atoms in naturally...Ch. 1 - The isotope of plutonium used for nuclear fission...Ch. 1 - The last “missing” element from the first six...Ch. 1 - Prob. 23PCh. 1 - In 1982, the production of a single atom of...Ch. 1 - Prob. 25PCh. 1 - Prob. 26PCh. 1 - Compute the relative molecular masses of the...Ch. 1 - Prob. 28PCh. 1 - Suppose that a person counts out gold atoms at the...Ch. 1 - A gold atom has a diameter of 2.881010m . Suppose...Ch. 1 - The vitamin A molecule has the formula C20H30O ,...Ch. 1 - Arrange the following in order of increasing mass:...Ch. 1 - Mercury is traded by the “flask,” a unit that has...Ch. 1 - Gold costs $400 per troy ounce, and...Ch. 1 - Aluminum oxide (Al2O3) occurs in nature as a...Ch. 1 - Prob. 36PCh. 1 - Soft wood chips weighing 17.2 kg are placed in an...Ch. 1 - In a reproduction of the Millikan oil-drop...Ch. 1 - A rough estimate of the radius of a nucleus is...Ch. 1 - In a neutron star, gravity causes the electrons to...Ch. 1 - Prob. 41APCh. 1 - Naturally occurring rubidium (Rb) consists of two...Ch. 1 - A sample of a gaseous binary compound of boron and...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardThe reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardWhat is the name of the major product formed during the reaction between benzoyl chloride and phenol? benzyl ester O phenyl benzoate ○ cyclopentanoate ○ benzyl phenoate ○ benzenecarboxylic acidarrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly.arrow_forward
- Provide the proper IUPAC name (only) for the following compound. Dashes, commas, and spaces must be used correctly. HO. OHarrow_forwardQuestion 2 0/1 pts Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly. HO CH 3 1-methyl-1-cyclohexanecarboxylic acidarrow_forwardPlease assign all the carbons for C-NMR and hydrogen for H-NMR. Please if I can get that less than hourarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Atomic Number, Atomic Mass, and the Atomic Structure | How to Pass ChemistryThe Nucleus: Crash Course Chemistry #1; Author: Crash Course;https://www.youtube.com/watch?v=FSyAehMdpyI;License: Standard YouTube License, CC-BY