
Concept explainers
(a)
Interpretation:
Lewis structures have to be drawn for the
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the
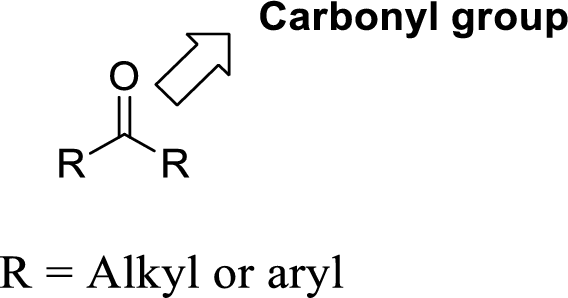
Carbonyl group:
A carbon atom is double-bonded to an oxygen atom
(b)
Interpretation:
Lewis structures have to be drawn for the functional group and valence electrons have to be shown.
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance. All the possible resonance structures are imaginary whereas the resonance hybrid is real. These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
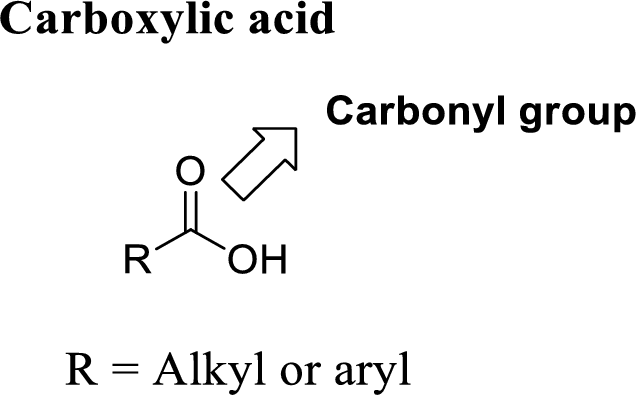
A carbon atom is double-bonded to an oxygen atom
If the carbonyl carbon is attached with hydroxyl group is called as carboxylic acid.
(c)
Interpretation:
Lewis structures have to be drawn for the functional group and valence electrons have to be shown.
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance. All the possible resonance structures are imaginary whereas the resonance hybrid is real. These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
Hydroxyl group:
Alcohol:
The compound contains hydroxyl
Example is given below
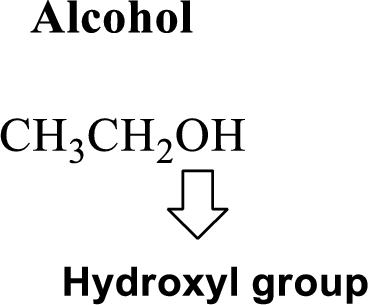
(d)
Interpretation:
Lewis structures have to be drawn for the functional group and valence electrons have to be shown.
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance. All the possible resonance structures are imaginary whereas the resonance hybrid is real. These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
Ester group:
A carbon atom is double-bonded to an oxygen atom
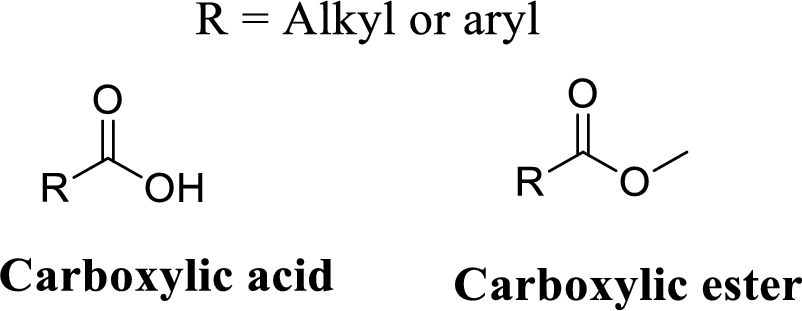
(e)
Interpretation:
Lewis structures have to be drawn for the functional group and valence electrons have to be shown.
Concept introduction:
Lewis structure: The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell. Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance. All the possible resonance structures are imaginary whereas the resonance hybrid is real. These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
Amide:
A carbon atom is double-bonded to an oxygen atom
If the carbonyl carbon is attached with nitrogen is called as amide.

Trending nowThis is a popular solution!

Chapter 1 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
