
Study Guide for Chemistry: The Central Science
14th Edition
ISBN: 9780134554075
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus, James C. Hill
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 10E
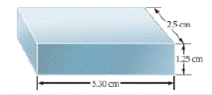
- How many significant figures should be reported for the volume of the metal bar shown here?
- If the mass of the bar is 104.72 g, how many significant figures should be reported when its density is determined using the calculated volume? [Section 1.60
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major products of the following organic reaction:
Some important notes:
Δ
CN
?
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
ONO reaction.
Click and drag to start drawing a structure.
The following product was made from diethyl ketone and what other reagent(s)?
£
HO
10
2-pentyne
1-butyne and NaNH2
☐ 1-propanol
☐ pyridine
butanal
☐ pentanoate
Which pair of reagents will form the given product?
OH
X
+
Y
a.
CH3
b.
CH2CH3
༧་་
C. CH3-
CH2CH3
d.o6.(རི॰
e.
CH3
OCH2CH3
-MgBr
f. CH3-MgBr
g. CH3CH2-MgBr
-C-CH3
CH2CH3
Chapter 1 Solutions
Study Guide for Chemistry: The Central Science
Ch. 1.2 - Practice Exercise 1 Which of the following is the...Ch. 1.2 - Aspirin is composed of 60.0% carbon, 4.5%...Ch. 1.5 - Practice Exercise 1 Which of the following weights...Ch. 1.5 - Practice Exercise 2 How many picometers are there...Ch. 1.5 - Practice Exercise 1 Using Wolfram Alpha...Ch. 1.5 - Practice Exercise 2 Ethylene glycol, the major...Ch. 1.5 - Practice Exercise 1 Platinum, Pt. is one of the...Ch. 1.5 - Practice Exercise 2 Calculate the density of a...Ch. 1.5 - Which of the following objects has the greatest...Ch. 1.5 - Prob. 1.5.2PE
Ch. 1.6 - Which of the following numbers in your personal...Ch. 1.6 - Practice Exercise 2 The back inside cover of the...Ch. 1.6 - Practice Exercise 1 An object is determined to...Ch. 1.6 - Practice Exercise 2 How many significant figures...Ch. 1.6 - Ellen recently purchased a new hybrid car and...Ch. 1.6 - Practice Exercise 2 It takes 10.5 s for a sprinter...Ch. 1.6 - Practice Exercise 1 You are asked to determine the...Ch. 1.6 - Practice Exercise 1 At a particular instant in...Ch. 1.7 - Practice Exercise 2 By using a conversion factor...Ch. 1.7 - Practice Exercise 1 Fabiola, who lives in Mexico...Ch. 1.7 - Practice Exercise 2 A car travels 28 mi per gallon...Ch. 1.7 - Practice Exercise 2 A car travels 28 mi per gallon...Ch. 1.7 - Practice Exercise 2 The surface area of Earth is...Ch. 1.7 - Practice Exercise 1 Composite decking is a...Ch. 1.7 - Practice Exercise 2 The density of the organic...Ch. 1.7 - Practice Exercise 2 If the mass of the container...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Musical instruments like trumpets and trombones...Ch. 1 - Consider the two spheres shown here, one made of...Ch. 1 - Is the separation method used in brewing a cup of...Ch. 1 - Identify each of the following as measurements of...Ch. 1 - Three spheres of equal size are composed of...Ch. 1 - The three targets from a rifle range shown below...Ch. 1 - What is the length of the pencil in the following...Ch. 1 - How many significant figures should be reported...Ch. 1 - Consider the jar of jelly beans in the photo. To...Ch. 1 - The photo below shows a picture of an agate stone....Ch. 1 - Classify each of the following as a pure substance...Ch. 1 - Classify each of the following as a pure substance...Ch. 1 - 1.15 Give the chemical symbol or name for the...Ch. 1 - 1.16 Give the chemical symbol or name for each of...Ch. 1 - A solid white substance A is heated strongly in...Ch. 1 - 1.18 You are hiking in the mountains and find a...Ch. 1 - 1.19 In the process of attempting to characterize...Ch. 1 - 1.20
Read the following description of the element...Ch. 1 - Prob. 21ECh. 1 - A match is lit and held under a cold piece of...Ch. 1 - Which separation method is better suited for...Ch. 1 - Two beakers contain clear, colorless liquids. When...Ch. 1 - Prob. 25ECh. 1 - Prob. 26ECh. 1 - Prob. 27ECh. 1 - Prob. 28ECh. 1 - Prob. 29ECh. 1 - Prob. 30ECh. 1 - 121 What exponential notation do the following...Ch. 1 -
1.32 Use appropriate metric prefixes to write the...Ch. 1 - Make the following conversions. 72 °F to °C, 216.7...Ch. 1 - a. The temperature on a warm summer day is 87 °F....Ch. 1 - Prob. 35ECh. 1 - A cube of osmium metal 1.500 cm on a side has a...Ch. 1 - To identify a liquid substance, a student...Ch. 1 - a. After the label fell off a bottle containing a...Ch. 1 - Prob. 39ECh. 1 -
1.40 Silicon for computer chips is grown in large...Ch. 1 - Prob. 41ECh. 1 - 1.42 A watt is a measure of power (the rate of...Ch. 1 - Indicate which of the following are exact numbers;...Ch. 1 - Indicate which of the following are exact numbers:...Ch. 1 - 1.45 What is the number of significant figures in...Ch. 1 - Indicate the number of significant figures in each...Ch. 1 - 1.47 Round each of the following numbers to four...Ch. 1 - 1.48
The diameter of Earth at the equator is 7926...Ch. 1 - Carry out the following operations and express the...Ch. 1 - Carry out the following operations and express the...Ch. 1 - You weigh an object on a balance and read the mass...Ch. 1 - You have a graduated cylinder that contains a...Ch. 1 - 153 Using your knowledge of metric units, English...Ch. 1 - 1.54 Using your knowledge of metric units, English...Ch. 1 - A bumblebee flies with a ground speed of 15.2 m/s....Ch. 1 - 1 56
a The speed of light in a vacuum is 2.998 x...Ch. 1 - Perform the following conversions: 5.00 days to s,...Ch. 1 - Carry out the following conversions: 0.105 in. to...Ch. 1 - How many liters of wine can be held in a wine...Ch. 1 - If an electric car is capable of going 225 km on a...Ch. 1 - The density of air at ordinary atmospheric...Ch. 1 - 1.62 The concentration of carbon monoxide in an...Ch. 1 - Prob. 63ECh. 1 - 1.64 A copper refinery produces a copper ingot...Ch. 1 - 165 Classify ea. al the folbwing as a pure...Ch. 1 - 1.66
Which is more likely to eventually be shown...Ch. 1 -
1.67 A sample of ascorbic acid (vitamin C) is...Ch. 1 - Prob. 68AECh. 1 - SO Two students deterrmne the percen.ge of lead in...Ch. 1 - 1.70
Is Om use of significant figures in ea. of...Ch. 1 - What type of quantity (for example, length,...Ch. 1 - 1.72 Give the derived SI units for each of the...Ch. 1 - 1.73 The distance from Earth to the Moon is...Ch. 1 - 1.74 Which of the following would you characterize...Ch. 1 -
1.75 The U.S. quarter has a mass of 5.67 g and is...Ch. 1 -
1.76 In the United States, water used for...Ch. 1 -
1.77 By using estimation techniques, determine...Ch. 1 - Suppose you decide to define your own temperature...Ch. 1 -
1.79 The liquid substances mercury (density =...Ch. 1 -
1.80 Two spheres of equal volume are placed on...Ch. 1 - Water has a density of 0.997 g/cm3 at 25C ; ice...Ch. 1 - A 32.65-g sample of a solid is placed in a flask....Ch. 1 - A thief plans to steal a gold sphere with a radius...Ch. 1 - Automobile batteries contain sulfuric acid, which...Ch. 1 - A 40-lb container of peat moss measures 14 x 20 x...Ch. 1 - A package of aluminum foil contains 50 ft2of foil,...Ch. 1 - Prob. 87AECh. 1 -
1.88 In 2005, J. Robin Warren and Barry J....Ch. 1 -
1 89 A 25 0-cm.long cylindrical glass tube,...Ch. 1 -
1.90 Gold is alloyed (mixed) with other metals to...Ch. 1 -
1.91 Paper chromatography is a simple but...Ch. 1 -
1.92 Judge the following statements as true or...Ch. 1 -
1.93 You are assigned the task of separating a...Ch. 1 - Prob. 94AE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
- Please draw by handarrow_forward3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY