You have just prepared a new substance in the lab and you wish to characterize its thermo- dynamic properties. As a start, you measure the vapor pressure of its solid and liquid phases at several temperatures between 200 and 400 K and you determine that the vapor pressures are accurately modeled by In G p(s) In 1 bar (₁ =) p(l) 1 bar = 18.000 = 11.500 3600 K T 2500 K T where T is in K. Calculate (a) the temperature (in K) and (b) the pressure (in bars) at the triple point.
You have just prepared a new substance in the lab and you wish to characterize its thermo- dynamic properties. As a start, you measure the vapor pressure of its solid and liquid phases at several temperatures between 200 and 400 K and you determine that the vapor pressures are accurately modeled by In G p(s) In 1 bar (₁ =) p(l) 1 bar = 18.000 = 11.500 3600 K T 2500 K T where T is in K. Calculate (a) the temperature (in K) and (b) the pressure (in bars) at the triple point.
Related questions
Question

Transcribed Image Text:You have just prepared a new substance in the lab and you wish to characterize its thermo-
dynamic properties. As a start, you measure the vapor pressure of its solid and liquid phases
at several temperatures between 200 and 400 K and you determine that the vapor pressures
are accurately modeled by
In
In
p(s)
1 bar
P(¹)
1 bar
= 18.000
= 11.500
3600 K
T
2500 K
T
where T is in K. Calculate (a) the temperature (in K) and (b) the pressure (in bars) at the triple
point.
Expert Solution
Step 1
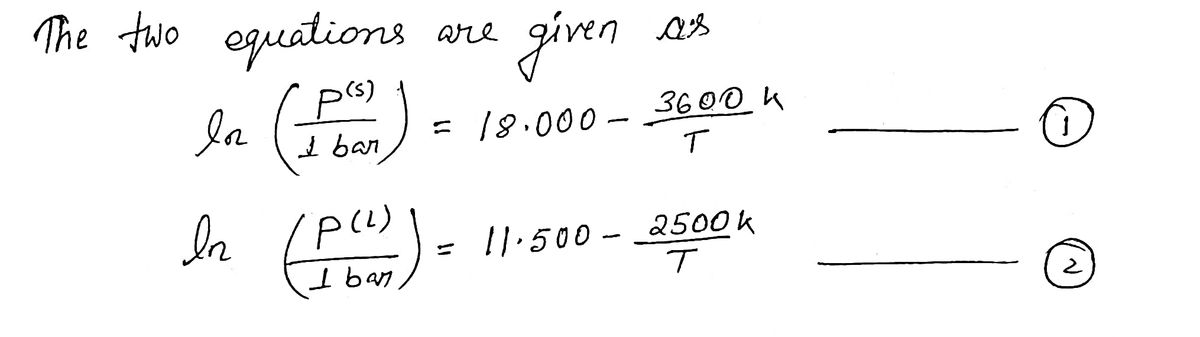
Step by step
Solved in 2 steps with 2 images
