You are an industrial chemist working for a company that makes methanol (CH3OH) according to the equilibrium reaction shown below. You love your job, except that your assistant does not have the best notebook keeping skills. One day you set up the reaction with the equilibrium concentrations shown below. CO (g) + 2 H2 (g) <--> CH3OH (g) 0.24 M 1.1 M 0.15 M at 327oC Later on your assistant mentions that he added some more CO,
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
You are an industrial chemist working for a company that makes methanol (CH3OH) according to the equilibrium reaction shown below. You love your job, except that your assistant does not have the best notebook keeping skills. One day you set up the reaction with the equilibrium concentrations shown below.
CO (g) + 2 H2 (g) <--> CH3OH (g)
0.24 M 1.1 M 0.15 M at 327oC
Later on your assistant mentions that he added some more CO, but he doesn't remember how much. But he also remembers that as the equilibrium was being reestablished, the hydrogen concentration changed by 0.3 M, although he doesn't remember if it went up by that much, or down by that much. How much carbon monoxide did he add, in M?
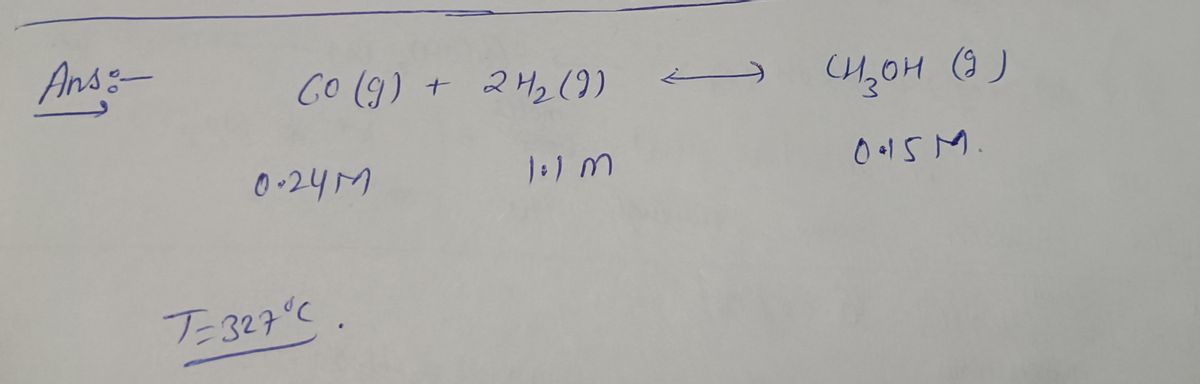
Step by step
Solved in 2 steps with 2 images

You are an industrial chemist working for a company that makes methanol (CH3OH) according to the equilibrium reaction shown below. You love your job, except that your assistant does not have the best notebook keeping skills. One day you set up the reaction with the equilibrium concentrations shown below.
CO (g) + 2 H2 (g) <--> CH3OH (g)
0.24 M 1.1 M 0.15 M at 327oC
Later on your assistant mentions that he added some more CO, but he doesn't remember how much. But he also remembers that as the equilibrium was being reestablished, the hydrogen concentration changed by 0.3 M, although he doesn't remember if it went up by that much, or down by that much. How much carbon monoxide did he add, in M?








