uld result for each pair and indicate I for Ensoluble, or S for soluble, or SS for slightly soluble. Two examples have been done. Prelab Table la : Prediction of Solubility of Salts in water. SO PO, OH- CF Br CO3²- C2H3O2 S²- CrO,²- Na+ S K* In - Fe²* FeClaFebr|Felk FeS DyFe(MFe(CH) Fe CO3F€CH30)} Fes FelrO I. I I. Cu²* CuClCubr す to
uld result for each pair and indicate I for Ensoluble, or S for soluble, or SS for slightly soluble. Two examples have been done. Prelab Table la : Prediction of Solubility of Salts in water. SO PO, OH- CF Br CO3²- C2H3O2 S²- CrO,²- Na+ S K* In - Fe²* FeClaFebr|Felk FeS DyFe(MFe(CH) Fe CO3F€CH30)} Fes FelrO I. I I. Cu²* CuClCubr す to
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Correct the ones that are marked wrong:

Transcribed Image Text:Prelab Table Ia: Using the solubility rules, write in the predicted results for each pair of
ions. Write the formula of the compound that would result for each pair and indicate I for
insoluble, or S for soluble, or SS for slightly soluble. Two examples have been done.
Prelab Table Ia : Prediction of Solubility of Salts in water.
PO-
CH
Br
SO?-
OH
CO3² | C2H3O: | S²-
CrO,?
Na+
S
K*
I.
-
Fe2+
I.
I.
I
I.
Cu²* CuClCubr
I.
Ag AC1
UI

Transcribed Image Text:Prelab Table Ib Solubility of Compounds in Water
Primary
Solubility in
100mL H20
from CRC
Solid
Name of
Predicted
species in
H20
Solubility in
H20
compound
compound
Handbook
1
BaSO4
Barium
BaSO4 (s)
Insoluble
0.31mg
sulfate
A(Pude(s) Insolubld D.2 ma
oluble 50.5 ma
carbonateoC0z (S) |nsolublel0. ma
ABCHLO: ilveracctatd or ore L.25mg
Ca3(PO4)2 Calcium
2
phosplate
(NH)»SO4 Ammonium o,
3
Tons
tead
4
P6CO3
Hg2Cl2
Mercury(I)
chloride
Hg2Cl2(s)
Insoluble
0.4 mg
7
Cu2O
CoppertiDoxide Cu,0(s) | Inolubld 0.2 mia
8.
C6H12O6
Glucore Joluble 0.212 x 10
Cattelou and Koor
ouble 5.834
sucrose
9
C6H5COOH
Benzoic acid
10
BaCl2
Barium chloride
Ba2+ and 2C
Soluble
37.0 g
ions
Expert Solution
Step 1
For Table 1a
Here SS= sparingly soluble
I= insoluble
S= soluble
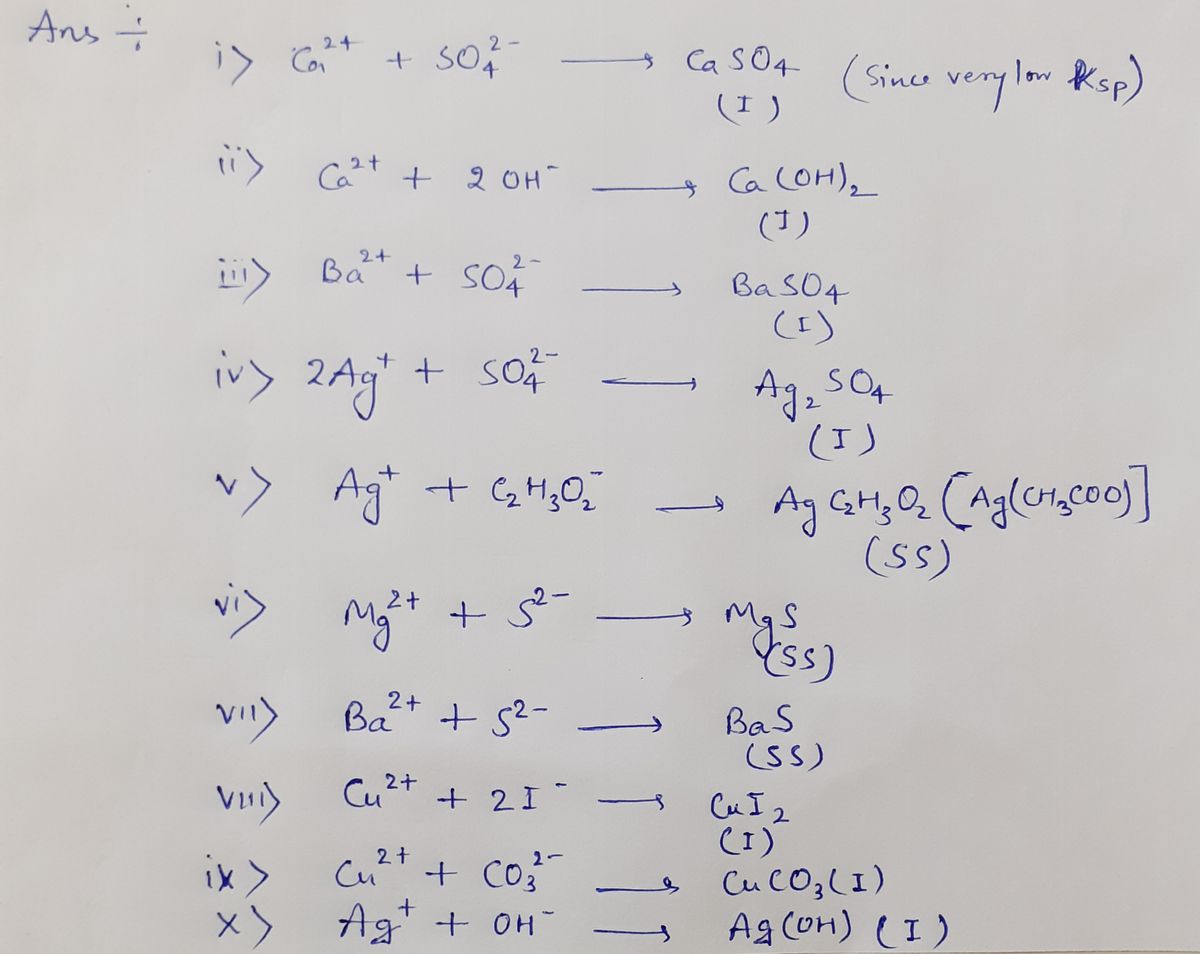
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY