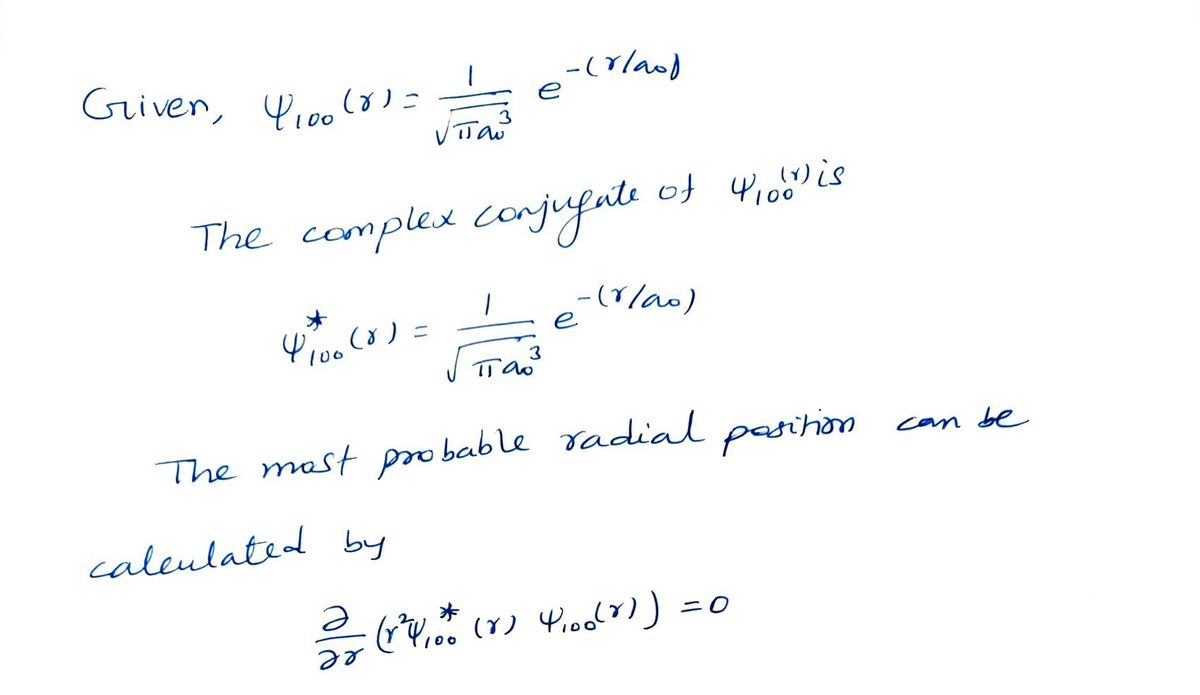
The normalised radial component of the wavefunction for the ground state of hydrogen is given by (n = 1,l = 0, m = 0) %3D 1 V100 (r) = -e(-r/ao) %3D 3 TaO where ao is the Bohr radius, qe is the electronic charge, u is the reduced mass and the other symbols have their standard meanings. Find an expression for the most probable radial position (in symbols).
The normalised radial component of the wavefunction for the ground state of hydrogen is given by (n = 1,l = 0, m = 0) %3D 1 V100 (r) = -e(-r/ao) %3D 3 TaO where ao is the Bohr radius, qe is the electronic charge, u is the reduced mass and the other symbols have their standard meanings. Find an expression for the most probable radial position (in symbols).
Related questions
Question

Transcribed Image Text:The normalised radial component of the wavefunction for the ground state of hydrogen is given by
(n = 1,l = 0, m = 0)
1
V100(r) =
ce(-r/ao)
%3D
3
V Tao
where ao
is the Bohr radius, qe is the electronic charge, u is the reduced mass and the other
symbols have their standard meanings.
Find an expression for the most probable radial position (in symbols).
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images
