The following series of reactions were carried out. PbCO:(s) + 2HN03(aq) → Pb(NO3)2(aq) + H2O(1) + CO2(g) Pb(NO:)2(aq) + 2HCI(aq) → 2HNO:(aq) + PbCl2(s) (a) If a student starts with 2.871 g of lead(II) carbonate for the first reaction and all other reagents are added in excess, what is the theoretical yield of lead(II) chloride solid? b) If the student isolates 2.385 g of lead(II) chloride, what is the percent yield?
The following series of reactions were carried out. PbCO:(s) + 2HN03(aq) → Pb(NO3)2(aq) + H2O(1) + CO2(g) Pb(NO:)2(aq) + 2HCI(aq) → 2HNO:(aq) + PbCl2(s) (a) If a student starts with 2.871 g of lead(II) carbonate for the first reaction and all other reagents are added in excess, what is the theoretical yield of lead(II) chloride solid? b) If the student isolates 2.385 g of lead(II) chloride, what is the percent yield?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.8: Product- Or Reactant-favored Reactions And Thermodynamics
Problem 2.1ACP
Related questions
Question
How do I solve this
![**Chemical Reactions and Yield Calculation**
The following series of reactions were carried out:
1. \( \text{PbCO}_3(s) + 2\text{HNO}_3(aq) \rightarrow \text{Pb(NO}_3)_2(aq) + \text{H}_2\text{O}(l) + \text{CO}_2(g) \)
2. \( \text{Pb(NO}_3)_2(aq) + 2\text{HCl}(aq) \rightarrow 2\text{HNO}_3(aq) + \text{PbCl}_2(s) \)
---
**Problem (a):**
If a student starts with 2.871 g of lead(II) carbonate (\( \text{PbCO}_3 \)) for the first reaction and all other reagents are added in excess, what is the theoretical yield of lead(II) chloride (\( \text{PbCl}_2 \)) solid?
**Problem (b):**
If the student isolates 2.385 g of lead(II) chloride, what is the percent yield?
---
### Explanation
To solve these problems, you will need to convert between moles and grams using molar masses and apply stoichiometry to determine theoretical and percent yields.
For **Problem (a)**, calculate the moles of \( \text{PbCO}_3 \) and use the stoichiometry of the given reactions to find the moles and then the grams of \( \text{PbCl}_2 \). This gives you the theoretical yield.
For **Problem (b)**, use the actual yield (2.385 g of \( \text{PbCl}_2 \)) and the theoretical yield from Problem (a) to determine the percent yield using the formula:
\[
\text{Percent Yield} = \left( \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \right) \times 100
\]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Faa8d3fce-d16d-4f4d-b492-1dee716a707b%2F1bce7c45-2a11-41b1-bfb6-35845907a5da%2Filtefck_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Chemical Reactions and Yield Calculation**
The following series of reactions were carried out:
1. \( \text{PbCO}_3(s) + 2\text{HNO}_3(aq) \rightarrow \text{Pb(NO}_3)_2(aq) + \text{H}_2\text{O}(l) + \text{CO}_2(g) \)
2. \( \text{Pb(NO}_3)_2(aq) + 2\text{HCl}(aq) \rightarrow 2\text{HNO}_3(aq) + \text{PbCl}_2(s) \)
---
**Problem (a):**
If a student starts with 2.871 g of lead(II) carbonate (\( \text{PbCO}_3 \)) for the first reaction and all other reagents are added in excess, what is the theoretical yield of lead(II) chloride (\( \text{PbCl}_2 \)) solid?
**Problem (b):**
If the student isolates 2.385 g of lead(II) chloride, what is the percent yield?
---
### Explanation
To solve these problems, you will need to convert between moles and grams using molar masses and apply stoichiometry to determine theoretical and percent yields.
For **Problem (a)**, calculate the moles of \( \text{PbCO}_3 \) and use the stoichiometry of the given reactions to find the moles and then the grams of \( \text{PbCl}_2 \). This gives you the theoretical yield.
For **Problem (b)**, use the actual yield (2.385 g of \( \text{PbCl}_2 \)) and the theoretical yield from Problem (a) to determine the percent yield using the formula:
\[
\text{Percent Yield} = \left( \frac{\text{Actual Yield}}{\text{Theoretical Yield}} \right) \times 100
\]
Expert Solution
Moles of PbCl2
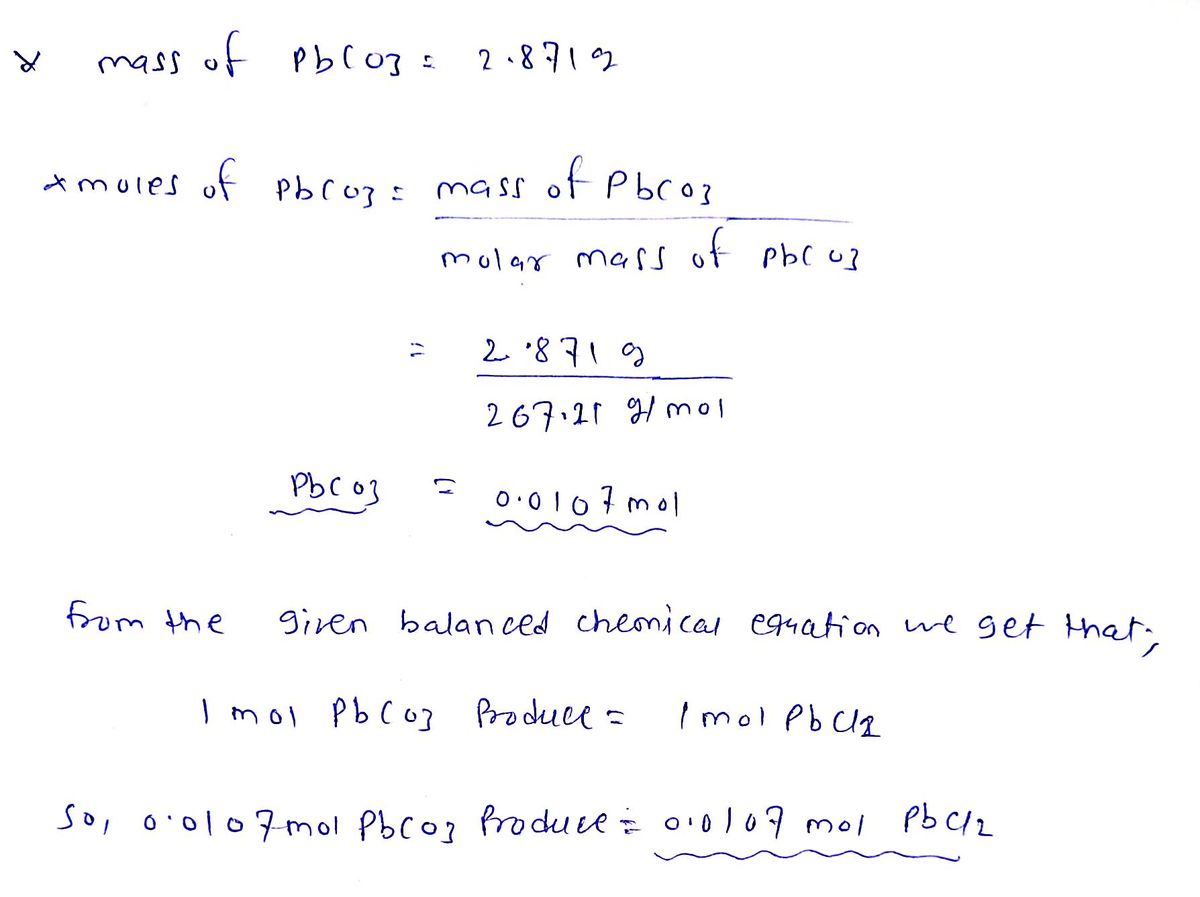
* Moles of Lead (ll) chloride produced = 0.0107 mol
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning