The equilibrium constant, Kp, for the following reaction is 0.160 at 298 K. 2NOBr(g)2NO(g) + Br₂(g) If an equilibrium mixture of the three gases in a 17.8 L container at 298 K contains NOBr at a pressure of 0.301 atm and NO at a pressure of 0.376 atm, the equilibrium partial pressure of Br₂ is atm. Submit Answer Retry Entire Group 9 more group attempts remaining
The equilibrium constant, Kp, for the following reaction is 0.160 at 298 K. 2NOBr(g)2NO(g) + Br₂(g) If an equilibrium mixture of the three gases in a 17.8 L container at 298 K contains NOBr at a pressure of 0.301 atm and NO at a pressure of 0.376 atm, the equilibrium partial pressure of Br₂ is atm. Submit Answer Retry Entire Group 9 more group attempts remaining
Related questions
Question
![eq
req
req
1req
2req
s 2req
#
3
20
The equilibrium constant, Kp, for the following reaction is 0.160 at 298 K.
2NOBr(g)2NO(g) + Br₂ (9)
If an equilibrium mixture of the three gases in a 17.8 L container at 298 K contains NOBr at a pressure of 0.301 atm
and NO at a pressure of 0.376 atm, the equilibrium partial pressure of Br₂ is
atm.
E
D
F3
C
Submit Answer
$
4
000
888
R
F
V
Show Hint
%
5
Retry Entire Group 9 more group attempts remaining
[References]
Use the References to access important values if needed for this question.
T
Cengage Learning Cengage Technical Support
FS
G
<<
6
B
MacBook Air
F6
Y
H
Topics]
&
7
N
U
J
*
00-
8
▶11
FB
1
M
(
9
K
O
V
of
)
O
L
F10
P
A.
command
:
F11
;
Previous
+
=
[
git
Next>
Save and Exit
B
option
F12](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3e5f0bee-bbde-421f-8347-e8b63f453811%2F98c5d0c5-a8cc-423c-aa6e-878427afa0b8%2Ff5uhr8_processed.jpeg&w=3840&q=75)
Transcribed Image Text:eq
req
req
1req
2req
s 2req
#
3
20
The equilibrium constant, Kp, for the following reaction is 0.160 at 298 K.
2NOBr(g)2NO(g) + Br₂ (9)
If an equilibrium mixture of the three gases in a 17.8 L container at 298 K contains NOBr at a pressure of 0.301 atm
and NO at a pressure of 0.376 atm, the equilibrium partial pressure of Br₂ is
atm.
E
D
F3
C
Submit Answer
$
4
000
888
R
F
V
Show Hint
%
5
Retry Entire Group 9 more group attempts remaining
[References]
Use the References to access important values if needed for this question.
T
Cengage Learning Cengage Technical Support
FS
G
<<
6
B
MacBook Air
F6
Y
H
Topics]
&
7
N
U
J
*
00-
8
▶11
FB
1
M
(
9
K
O
V
of
)
O
L
F10
P
A.
command
:
F11
;
Previous
+
=
[
git
Next>
Save and Exit
B
option
F12
Expert Solution
Step 1
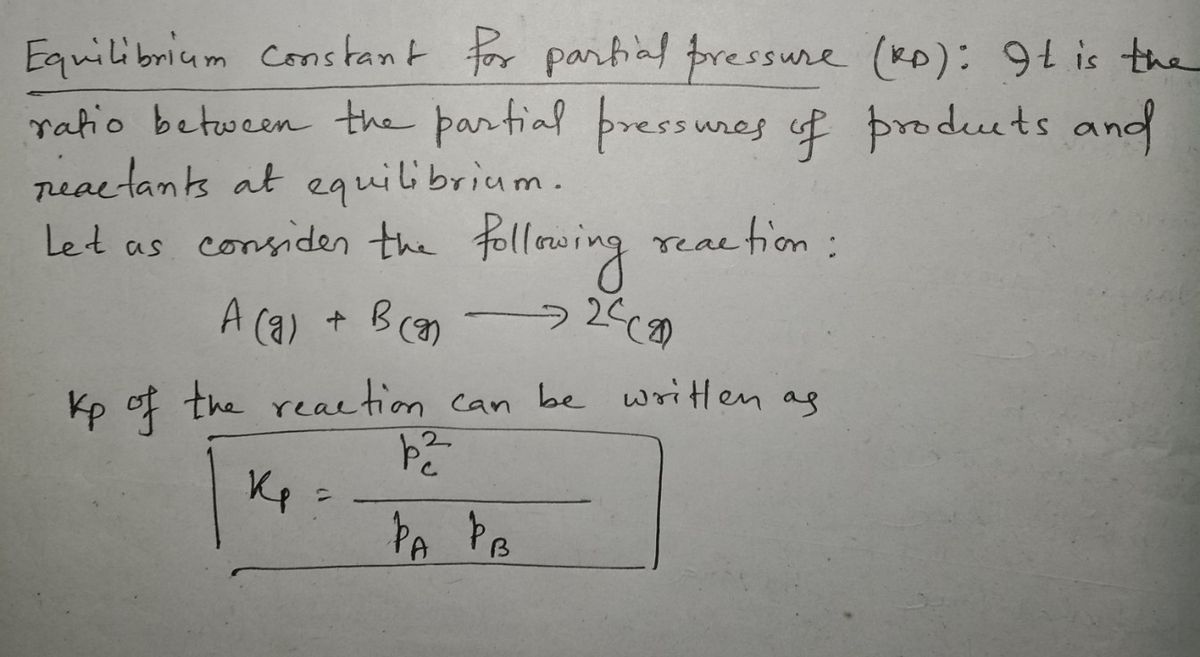
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.