The equilibrium-constant expression The equilibrium-constant expression i used to describe the concentration of reactants and products for a reaction in dynamic equilibrium. For ideal gases and ideal solutions in homogeneous equilibria, where all reactants and products are in the same phase, the extent to which a particular chemical reaction proceeds to products is given by the equilibrium equation [C] [D]d K = [A][B] ▼ where K is the equilibrium constant and the right-hand side of the equation is known as the equilibrium-constant expression. The concentration of each product raised to its coefficient is divided by the concentration of each reagent raised to its coefficient according the the balanced chemical equation. Therefore, the higher the concentration of products, the larger the value of K will be. Part A Identify the proper form of the equilibrium-constant expression for the equation ▸View Available Hint(s) Ok= Ok= Ok= O K = [Nz]O [NO]² Submit [NO] [N₂][0₂] Part B [NO]² [N₂][0₂] 2 [NO] [N₂][0₂] The equilibrium-constant of the reaction aA+bB cC+ dD. is K= 2.1 x 10-20. What can be said about this reaction? ▸ View Available Hint(s) N₂(g) + O₂(g) = 2NO(g) Review I Constants I Periodic Table NO₂(g) + NO3(g) = N₂O5 (g) O At equilibrium the concentration of products and reactants is about the same. O At equilibrium the concentration of products is much greater than the concentration of reactants. O At equilibrium the concentration of reactants is much greater than that of products. O There are no reactants left over once the reaction reaches equilibrium.
The equilibrium-constant expression The equilibrium-constant expression i used to describe the concentration of reactants and products for a reaction in dynamic equilibrium. For ideal gases and ideal solutions in homogeneous equilibria, where all reactants and products are in the same phase, the extent to which a particular chemical reaction proceeds to products is given by the equilibrium equation [C] [D]d K = [A][B] ▼ where K is the equilibrium constant and the right-hand side of the equation is known as the equilibrium-constant expression. The concentration of each product raised to its coefficient is divided by the concentration of each reagent raised to its coefficient according the the balanced chemical equation. Therefore, the higher the concentration of products, the larger the value of K will be. Part A Identify the proper form of the equilibrium-constant expression for the equation ▸View Available Hint(s) Ok= Ok= Ok= O K = [Nz]O [NO]² Submit [NO] [N₂][0₂] Part B [NO]² [N₂][0₂] 2 [NO] [N₂][0₂] The equilibrium-constant of the reaction aA+bB cC+ dD. is K= 2.1 x 10-20. What can be said about this reaction? ▸ View Available Hint(s) N₂(g) + O₂(g) = 2NO(g) Review I Constants I Periodic Table NO₂(g) + NO3(g) = N₂O5 (g) O At equilibrium the concentration of products and reactants is about the same. O At equilibrium the concentration of products is much greater than the concentration of reactants. O At equilibrium the concentration of reactants is much greater than that of products. O There are no reactants left over once the reaction reaches equilibrium.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter18: Chemical Equilibrium
Section: Chapter Questions
Problem 17E
Related questions
Question
100%
31. Please see attached image
![The equilibrium-constant expression
The equilibrium-constant expression i used to describe the concentration of reactants and products for a reaction in dynamic equilibrium. For ideal gases and ideal solutions in homogeneous equilibria, where all reactants and
products are in the same phase, the extent to which a particular chemical reaction proceeds to products is given by the equilibrium equation
▼
Part A
Identify the proper form of the equilibrium-constant expression for the equation
where K is the equilibrium constant and the right-hand side of the equation is known as the equilibrium-constant expression.
The concentration of each product raised to its coefficient is divided by the concentration of each reagent raised to its coefficient according the the balanced chemical equation. Therefore, the higher the concentration of products,
the larger the value of K will be.
▸ View Available Hint(s)
OK=
Ok=
Ok=
O K = [Nz]O,
[NO]²
Submit
[NO]
[N₂][0₂]
Part B
[NO]²
[N₂][0₂]
2[NO]
[N₂][0₂]
The equilibrium-constant of the reaction
aA+bB cC+ dD.
is K= 2.1 x 10-20. What can be said about this reaction?
View Available Hint(s)
K =
[C] [D]d
[A][B]
N₂(g) + O₂(g) = 2NO(g)
O At equilibrium the concentration of products and reactants is about the same.
O At equilibrium the concentration of products is much greater than the concentration of reactants.
O At equilibrium the concentration of reactants is much greater than that of products.
O There are no reactants left over once the reaction reaches equilibrium.
Review I Constants I Periodic Table
NO₂(g) + NO3(g) = N₂O5 (g)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F1205cd08-0e41-4ad8-bd1c-6b97372ee2c1%2F96d8f074-91e7-4476-8be6-8dc01c1702ba%2Fzbsw87z_processed.jpeg&w=3840&q=75)
Transcribed Image Text:The equilibrium-constant expression
The equilibrium-constant expression i used to describe the concentration of reactants and products for a reaction in dynamic equilibrium. For ideal gases and ideal solutions in homogeneous equilibria, where all reactants and
products are in the same phase, the extent to which a particular chemical reaction proceeds to products is given by the equilibrium equation
▼
Part A
Identify the proper form of the equilibrium-constant expression for the equation
where K is the equilibrium constant and the right-hand side of the equation is known as the equilibrium-constant expression.
The concentration of each product raised to its coefficient is divided by the concentration of each reagent raised to its coefficient according the the balanced chemical equation. Therefore, the higher the concentration of products,
the larger the value of K will be.
▸ View Available Hint(s)
OK=
Ok=
Ok=
O K = [Nz]O,
[NO]²
Submit
[NO]
[N₂][0₂]
Part B
[NO]²
[N₂][0₂]
2[NO]
[N₂][0₂]
The equilibrium-constant of the reaction
aA+bB cC+ dD.
is K= 2.1 x 10-20. What can be said about this reaction?
View Available Hint(s)
K =
[C] [D]d
[A][B]
N₂(g) + O₂(g) = 2NO(g)
O At equilibrium the concentration of products and reactants is about the same.
O At equilibrium the concentration of products is much greater than the concentration of reactants.
O At equilibrium the concentration of reactants is much greater than that of products.
O There are no reactants left over once the reaction reaches equilibrium.
Review I Constants I Periodic Table
NO₂(g) + NO3(g) = N₂O5 (g)
Expert Solution
Step 1
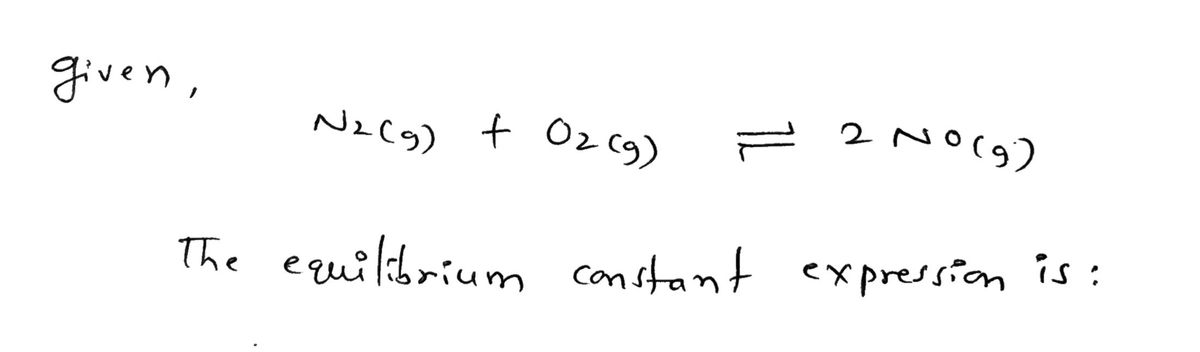
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning