Suppose 65.0 g of K(s) reacts with 25.0 g oxygen gas to produce 54.7 g of potassium oxide. WHat is the % yield. (In class we got 70.0%). When solving could you go over in this problem by teaching me what the actual yield, theorictical yield, and percent yield are and how to find each one of those. It really confuses me which one is which and how to solve for each of them.
Suppose 65.0 g of K(s) reacts with 25.0 g oxygen gas to produce 54.7 g of potassium oxide. WHat is the % yield. (In class we got 70.0%). When solving could you go over in this problem by teaching me what the actual yield, theorictical yield, and percent yield are and how to find each one of those. It really confuses me which one is which and how to solve for each of them.
Percent yield is defined as the percentage ratio of the actual yield to the theoretical yield multiplied by 100%. i.e.
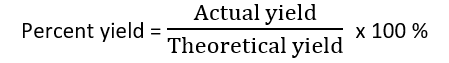
Now,actual yield refers to the amount of product actually produced during the reaction and theoretical yield refers to the resultant amount of product that can be produced from the amount of limiting reagent available in the reaction.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images









