Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Question 21**
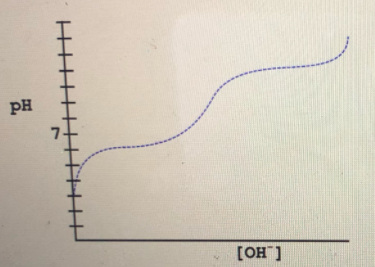
Report the largest pK for the weak acid whose titration curve is shown.
**Graph Description:**
The graph displays a titration curve of a weak acid. The x-axis is labeled with the concentration of hydroxide ions \([OH^-]\), and the y-axis is labeled with pH values. The curve begins at a pH below 7 and slopes upwards, showing an increase in pH as \([OH^-]\) increases. It has distinct regions, including a buffer region (where the curve flattens), indicating the pKa, where the weak acid is halfway neutralized. After this point, the curve shows a steeper increase in pH as more base is added.
Please report the largest pKa value observed in this titration curve.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F72a720b9-38b2-4beb-ae1b-56a99a6fccf3%2F2da7f986-acc9-46c2-b1f4-3e678a69735b%2Fv78qanh_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Question 21**
Report the largest pK for the weak acid whose titration curve is shown.
**Graph Description:**
The graph displays a titration curve of a weak acid. The x-axis is labeled with the concentration of hydroxide ions \([OH^-]\), and the y-axis is labeled with pH values. The curve begins at a pH below 7 and slopes upwards, showing an increase in pH as \([OH^-]\) increases. It has distinct regions, including a buffer region (where the curve flattens), indicating the pKa, where the weak acid is halfway neutralized. After this point, the curve shows a steeper increase in pH as more base is added.
Please report the largest pKa value observed in this titration curve.
Expert Solution
Step 1
The titration curve given is,
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY