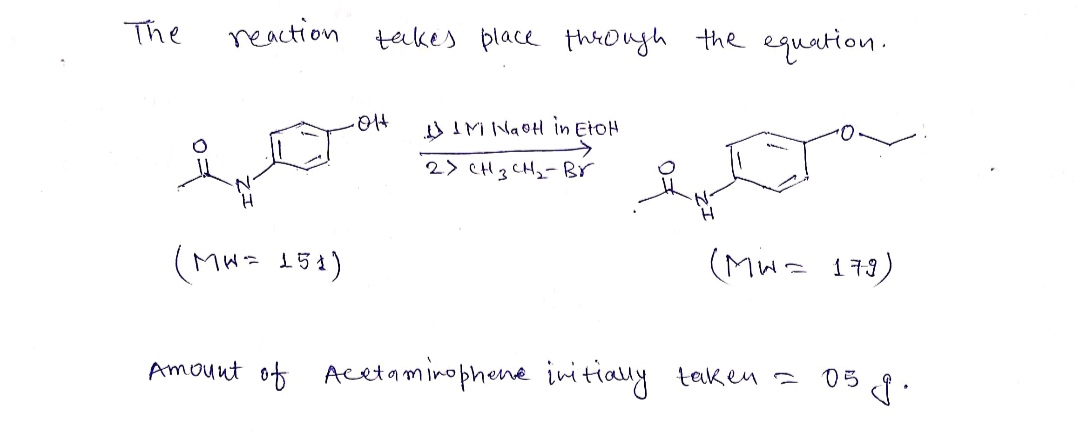
Reaction: A Williamson ether synthesis' involves an alcohol and an alkyl halide reacting to form an ether. The first step is the deprotonation of the alcohol using a sufficiently strong base. In this experiment, the base used will be sodium hydroxide which has been dissolved in ethanol. The resulting alkoxide, a phenoxide in this case, attacks the alkyl halide in an SN2 fashion. The final product, phenacetin, is soluble in ethanol but insoluble in water, which will allow it to be purified before analysis. OH 1. 1M NaOH in EtOH H 2. СH3CH2-Br H Na* Br
Reactions of Ethers
Ethers (R-O-R’) are compounds formed by replacing hydrogen atoms of an alcohol (R-OH compound) or a phenol (C6H5OH) by an aryl/ acyl group (functional group after removing single hydrogen from an aromatic ring). In this section, reaction, preparation and behavior of ethers are discussed in the context of organic chemistry.
Epoxides
Epoxides are a special class of cyclic ethers which are an important functional group in organic chemistry and generate reactive centers due to their unusual high reactivity. Due to their high reactivity, epoxides are considered to be toxic and mutagenic.
Williamson Ether Synthesis
An organic reaction in which an organohalide and a deprotonated alcohol forms ether is known as Williamson ether synthesis. Alexander Williamson developed the Williamson ether synthesis in 1850. The formation of ether in this synthesis is an SN2 reaction.
Can you answer this question and show all your work clearly please.
- Question: Calculate the theoretical yield for this reaction.
Part 1.
- Add 05 g of acetaminophen to a 25 mL round bottom flask
- clamp this flask to a ring stand so that it can remain suspended in the air before you do anything else
- Add a stir bar to the flask
- Add 8 mL of the 1M ethanolic NaOH to the flask.
- Begin stirring the solution
- Equip the flask with a reflux condenser with water flowing
- Put the reflux condenser onto the ring stand with a second clamp
- Water flows in through the bottom and out through the top
- Reflux the solution for 15 min
- Start the hot plate at 120 C
- After 15 min, raise the flask and condenser a few inches above the hot plate so that it can cool
Part 2.
- Add 0.75 mL of bromoethane to the reaction by pouring it through the condenser
- Reflux the solution for an additional 15 min
- After the solution has finished refluxing, allow it to cool enough to handle.
- Obtain a TLC plate and set up 3 lanes
- spot a sample of the acetaminophen provided in lane 1
- spot a sample of this reaction mixture in lane 2
- Pour the solution into a beaker containing 10 mL of ice and 10 mL of water.
- if the solution is still warm, cool it on an ice bath
- Collect the solid that forms by vacuum filtration
Part 3.
- Recrystallize the crude solid from ethanol and water
- Collect the final solid by vacuum filtration
- allow the solid to dry over air
- take a small amount of solid, dissolve it in ethanol and spot it in lane 3 of your TLC plate

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









