Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
See image below

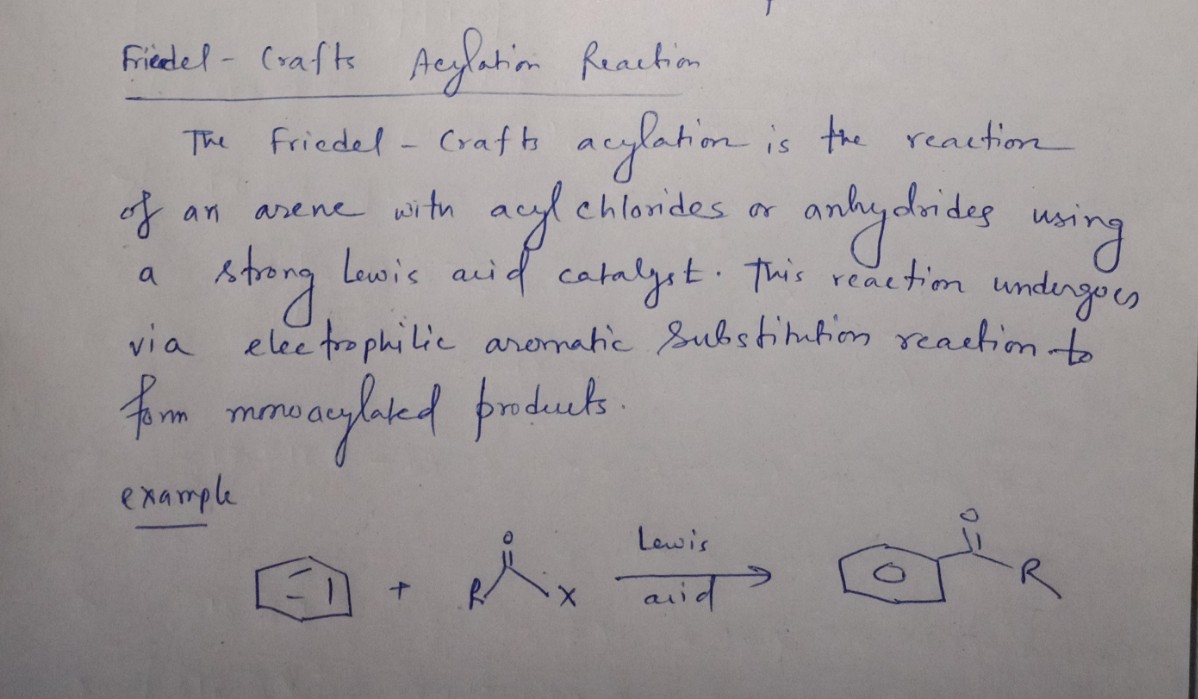
Transcribed Image Text:### Reaction Overview
The image depicts a chemical reaction where benzene reacts with benzoyl chloride in the presence of aluminum chloride (AlCl₃) as a catalyst. The task is to draw the major organic product of this reaction, ignoring any inorganic byproducts.
### Reactants
1. **Benzene (C₆H₆):** Represented by a hexagon with alternating double bonds, indicating its aromatic structure.
2. **Benzoyl Chloride (C₆H₅COCl):** Composed of a benzene ring attached to a carbonyl group (C=O), which is connected to a chlorine atom (Cl).
### Reaction Conditions
- **Catalyst:** Aluminum chloride (AlCl₃) is used to facilitate the reaction.
### Reaction Type
This is a Friedel-Crafts acylation reaction, where an acyl group is introduced to the benzene ring.
### Expected Product
The Friedel-Crafts acylation will result in the attachment of the benzoyl group (C₆H₅CO-) to the benzene ring, forming benzophenone (C₆H₅COC₆H₅) as the major product.
### Mechanism Summary
1. **Formation of Acyl Cation:** AlCl₃ reacts with the chlorine atom in benzoyl chloride, helping to generate the acyl cation (C₆H₅C⁺=O).
2. **Electrophilic Aromatic Substitution:** The acyl cation acts as an electrophile and reacts with the benzene ring to form the acylated product.
This reaction exemplifies a key transformation in organic chemistry, used widely in the synthesis of aromatic ketones.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY