Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
You have the Major product it makes, so what is the product it comes from... There isn't much explanation to this... it's either you know it or you dont, and if you dont know it please pass it on the next person... I'm getting really tired of people declining questions because they dont know how to do it and they waste my time... I wait about 6-8 hours for my ONE QUESTION to get either answered or on Bartleby it mostly just Declinded and thats honestly such a waste of my time when you could have justed "SKIPPED IT" and let whoever comes in the next shift to see if they know how.. like For real...

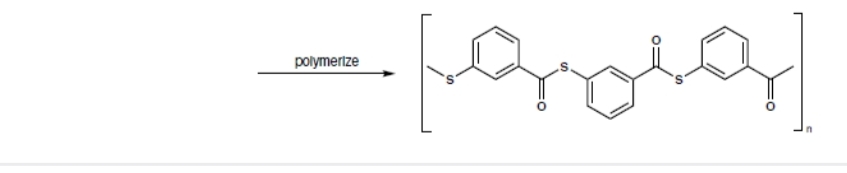
Transcribed Image Text:The image illustrates the polymerization process, showing the structure of a polymer formed through the reaction.
**Description:**
- **Polymerization Reaction:**
- The arrow labeled "polymerize" indicates the chemical reaction where smaller monomer units are chemically bonded to form a polymer.
- **Polymer Structure:**
- The repeating unit enclosed within brackets represents the structural formula of the polymer.
- The structure contains aromatic rings connected by sulfur (S) and oxygen (O) atoms, along with carbonyl groups (C=O).
- The repeating unit includes:
- Thiophene rings: Five-membered rings containing sulfur.
- Phenyl groups: Six-membered aromatic rings.
- Carbonyl linkages: Connecting the aromatic units.
- A subscript "n" denotes that this unit repeats n times, indicating the polymer chain can extend with many repeating units.
This diagram provides insight into the basic chemical structure of the polymer, illustrating the backbone and recurring units.
Expert Solution
Step 1: Polymer
The given polymer is shown below.
We have to determine the monomer structure.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY