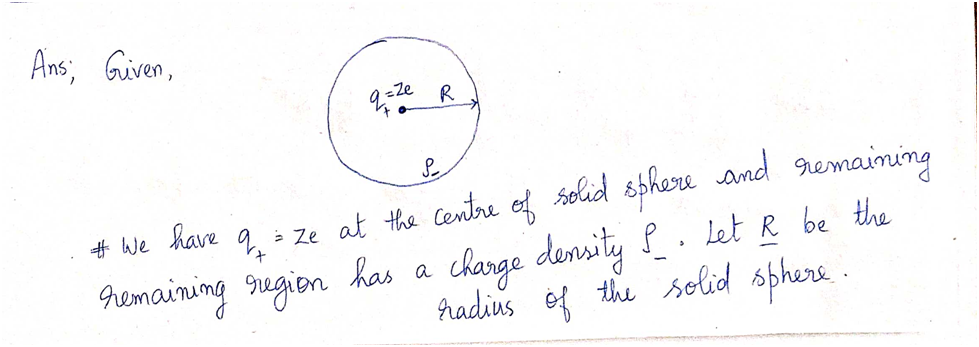
**Problem 2:** An early model of the atom, proposed by Rutherford after his discovery of the atomic nucleus, had a positive point charge \(+Ze\) (the nucleus) at the center of a sphere of radius \(R\) with uniformly distributed negative charge \(-Ze\). \(Z\) is the atomic number, the number of protons in the nucleus, and the number of electrons in the negative sphere. Show that the electric field strength inside this atom is \[ E_{\text{in}} = \frac{Ze}{4\pi\varepsilon_0}\left(\frac{1}{r^2} - \frac{r}{R^3}\right). \] **a)** Fig. 2 illustrates the model of the atom. In the figure, draw the Gaussian surface that you will use to find the field. Draw the vectors that represent the electric field at the Gaussian surface. How do you know the direction of the electric field inside the atom? --- **Fig. 2:** The scheme for Problem 2 Diagram explanation: The figure shows a circle representing a sphere with a radius \(R\). A small dot in the center represents the positive charge \(Q_+ = Ze\). Around this center, is a dashed line indicating the Gaussian surface. The electric field vectors would point away from the center along the Gaussian surface. --- **b)** What is the volume charge density \(\rho_-\) of the negative sphere? Use Gauss's law and the symmetry arguments in order to find the expression for the electric field strength inside the atom. What is the electric field strength outside this atom? **c)** A uranium atom has \(Z = 92\) and \(R = 0.10 \, \text{nm}\). What is the electric field strength at \(r = R/2\)?
**Problem 2:** An early model of the atom, proposed by Rutherford after his discovery of the atomic nucleus, had a positive point charge \(+Ze\) (the nucleus) at the center of a sphere of radius \(R\) with uniformly distributed negative charge \(-Ze\). \(Z\) is the atomic number, the number of protons in the nucleus, and the number of electrons in the negative sphere. Show that the electric field strength inside this atom is \[ E_{\text{in}} = \frac{Ze}{4\pi\varepsilon_0}\left(\frac{1}{r^2} - \frac{r}{R^3}\right). \] **a)** Fig. 2 illustrates the model of the atom. In the figure, draw the Gaussian surface that you will use to find the field. Draw the vectors that represent the electric field at the Gaussian surface. How do you know the direction of the electric field inside the atom? --- **Fig. 2:** The scheme for Problem 2 Diagram explanation: The figure shows a circle representing a sphere with a radius \(R\). A small dot in the center represents the positive charge \(Q_+ = Ze\). Around this center, is a dashed line indicating the Gaussian surface. The electric field vectors would point away from the center along the Gaussian surface. --- **b)** What is the volume charge density \(\rho_-\) of the negative sphere? Use Gauss's law and the symmetry arguments in order to find the expression for the electric field strength inside the atom. What is the electric field strength outside this atom? **c)** A uranium atom has \(Z = 92\) and \(R = 0.10 \, \text{nm}\). What is the electric field strength at \(r = R/2\)?
Related questions
Question
Hello, I need help with PART A, PART B, AND PART C, I don't know how to do these three problems can you help me because it will help to see why I got the answer wrong. Also, can you label which one is Part A, PART B AND PART C
![**Problem 2:**
An early model of the atom, proposed by Rutherford after his discovery of the atomic nucleus, had a positive point charge \(+Ze\) (the nucleus) at the center of a sphere of radius \(R\) with uniformly distributed negative charge \(-Ze\). \(Z\) is the atomic number, the number of protons in the nucleus, and the number of electrons in the negative sphere. Show that the electric field strength inside this atom is
\[ E_{\text{in}} = \frac{Ze}{4\pi\varepsilon_0}\left(\frac{1}{r^2} - \frac{r}{R^3}\right). \]
**a)** Fig. 2 illustrates the model of the atom. In the figure, draw the Gaussian surface that you will use to find the field. Draw the vectors that represent the electric field at the Gaussian surface. How do you know the direction of the electric field inside the atom?
---
**Fig. 2:** The scheme for Problem 2
Diagram explanation: The figure shows a circle representing a sphere with a radius \(R\). A small dot in the center represents the positive charge \(Q_+ = Ze\). Around this center, is a dashed line indicating the Gaussian surface. The electric field vectors would point away from the center along the Gaussian surface.
---
**b)** What is the volume charge density \(\rho_-\) of the negative sphere? Use Gauss's law and the symmetry arguments in order to find the expression for the electric field strength inside the atom. What is the electric field strength outside this atom?
**c)** A uranium atom has \(Z = 92\) and \(R = 0.10 \, \text{nm}\). What is the electric field strength at \(r = R/2\)?](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F135ae25c-4e57-47fe-981c-440929c4b953%2F7639bbe2-0369-4f58-9327-ad6224a429fd%2F35og3nh_processed.png&w=3840&q=75)
Transcribed Image Text:**Problem 2:**
An early model of the atom, proposed by Rutherford after his discovery of the atomic nucleus, had a positive point charge \(+Ze\) (the nucleus) at the center of a sphere of radius \(R\) with uniformly distributed negative charge \(-Ze\). \(Z\) is the atomic number, the number of protons in the nucleus, and the number of electrons in the negative sphere. Show that the electric field strength inside this atom is
\[ E_{\text{in}} = \frac{Ze}{4\pi\varepsilon_0}\left(\frac{1}{r^2} - \frac{r}{R^3}\right). \]
**a)** Fig. 2 illustrates the model of the atom. In the figure, draw the Gaussian surface that you will use to find the field. Draw the vectors that represent the electric field at the Gaussian surface. How do you know the direction of the electric field inside the atom?
---
**Fig. 2:** The scheme for Problem 2
Diagram explanation: The figure shows a circle representing a sphere with a radius \(R\). A small dot in the center represents the positive charge \(Q_+ = Ze\). Around this center, is a dashed line indicating the Gaussian surface. The electric field vectors would point away from the center along the Gaussian surface.
---
**b)** What is the volume charge density \(\rho_-\) of the negative sphere? Use Gauss's law and the symmetry arguments in order to find the expression for the electric field strength inside the atom. What is the electric field strength outside this atom?
**c)** A uranium atom has \(Z = 92\) and \(R = 0.10 \, \text{nm}\). What is the electric field strength at \(r = R/2\)?
Expert Solution
Step 1
Step by step
Solved in 4 steps with 4 images
