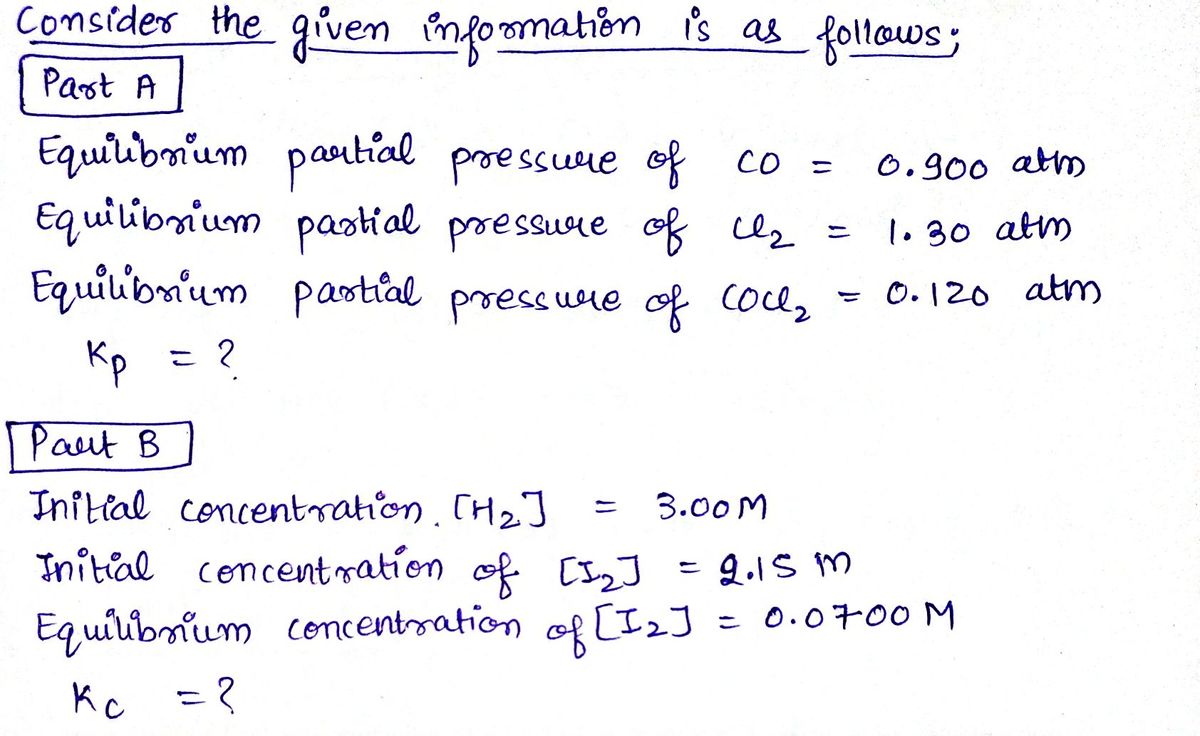
Part A Phosgene (carbonyl chloride), COCI₂, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. Phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: CO(g) + Cl₂(g) = COC1₂(g) Carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 452 °C. At equilibrium, the concentrations were measured and the following results obtained: What is the equilibrium constant, Kp. of this reaction? Express your answer numerically. ▸ View Available Hint(s) Kp = Submit ΨΕ ΑΣΦΑ → Previous Answers Gas CO Cl₂ | COC, BO ? Partial Pressure (atm) 0.900 1.30 0.120
Part A Phosgene (carbonyl chloride), COCI₂, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. Phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: CO(g) + Cl₂(g) = COC1₂(g) Carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 452 °C. At equilibrium, the concentrations were measured and the following results obtained: What is the equilibrium constant, Kp. of this reaction? Express your answer numerically. ▸ View Available Hint(s) Kp = Submit ΨΕ ΑΣΦΑ → Previous Answers Gas CO Cl₂ | COC, BO ? Partial Pressure (atm) 0.900 1.30 0.120
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Calculating Equilibrium Constants**
The equilibrium constant, \( K \), of a reaction at a particular temperature is determined by the concentrations or pressures of the reactants and products at equilibrium.
For a gaseous reaction with the general form:
\[ aA + bB \rightleftharpoons cC + dD \]
the \( K_c \) and \( K_p \) expressions are given by:
\[ K_c = \frac{{[C]^c[D]^d}}{{[A]^a[B]^b}} \]
\[ K_p = \frac{{(P_C)^c(P_D)^d}}{{(P_A)^a(P_B)^b}} \]
The subscript c or p indicates whether \( K \) is expressed in terms of concentrations or pressures. Equilibrium-constant expressions do not include a term for any pure solids or liquids that may be involved since their composition does not change throughout the reaction. The standard state of a pure substance is the pure substance itself, and although the quantity may change, the sample remains pure. The constant value is incorporated into the value of \( K \), and does not need to be accounted for separately.
---
### Part A
Phosgene (carbonyl chloride), \( \text{COCl}_2 \), is an extremely toxic gas used in manufacturing certain dyes and plastics. Phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures:
\[ \text{CO}(g) + \text{Cl}_2(g) \rightleftharpoons \text{COCl}_2(g) \]
Carbon monoxide and chlorine gas are allowed to react in a sealed vessel at \( 452 \, ^\circ \text{C} \). At equilibrium, the concentrations were measured and the following results obtained:
| Gas | Partial Pressure (atm) |
|-------|------------------------|
| CO | 0.900 |
| \( \text{Cl}_2 \) | 1.30 |
| \( \text{COCl}_2 \) | 0.120 |
What is the equilibrium constant, \( K_p \), of this reaction?
Express your answer numerically.
[View Available Hint(s)]
\( K_p = \)
---
(There is also an interactive input box to enter the \( K_p \) value, accompanied by options to format the text and a submit button.)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F06ef5119-c7b9-4136-ae1c-12b1af17aba5%2F9a4c22e8-127f-4da6-994a-a0a299387dbb%2Frhoqh1e_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Calculating Equilibrium Constants**
The equilibrium constant, \( K \), of a reaction at a particular temperature is determined by the concentrations or pressures of the reactants and products at equilibrium.
For a gaseous reaction with the general form:
\[ aA + bB \rightleftharpoons cC + dD \]
the \( K_c \) and \( K_p \) expressions are given by:
\[ K_c = \frac{{[C]^c[D]^d}}{{[A]^a[B]^b}} \]
\[ K_p = \frac{{(P_C)^c(P_D)^d}}{{(P_A)^a(P_B)^b}} \]
The subscript c or p indicates whether \( K \) is expressed in terms of concentrations or pressures. Equilibrium-constant expressions do not include a term for any pure solids or liquids that may be involved since their composition does not change throughout the reaction. The standard state of a pure substance is the pure substance itself, and although the quantity may change, the sample remains pure. The constant value is incorporated into the value of \( K \), and does not need to be accounted for separately.
---
### Part A
Phosgene (carbonyl chloride), \( \text{COCl}_2 \), is an extremely toxic gas used in manufacturing certain dyes and plastics. Phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures:
\[ \text{CO}(g) + \text{Cl}_2(g) \rightleftharpoons \text{COCl}_2(g) \]
Carbon monoxide and chlorine gas are allowed to react in a sealed vessel at \( 452 \, ^\circ \text{C} \). At equilibrium, the concentrations were measured and the following results obtained:
| Gas | Partial Pressure (atm) |
|-------|------------------------|
| CO | 0.900 |
| \( \text{Cl}_2 \) | 1.30 |
| \( \text{COCl}_2 \) | 0.120 |
What is the equilibrium constant, \( K_p \), of this reaction?
Express your answer numerically.
[View Available Hint(s)]
\( K_p = \)
---
(There is also an interactive input box to enter the \( K_p \) value, accompanied by options to format the text and a submit button.)
![**Understanding Equilibrium Constants**
The equilibrium constant, \( K \), of a reaction at a particular temperature is determined by the concentrations or pressures of the reactants and products at equilibrium.
**For a gaseous reaction with the general form:**
\[ aA + bB \rightleftharpoons cC + dD \]
**The expressions for \( K_c \) and \( K_p \) are:**
\[ K_c = \frac{[C]^c[D]^d}{[A]^a[B]^b} \]
\[ K_p = \frac{(P_C)^c(P_D)^d}{(P_A)^a(P_B)^b} \]
Here, the subscript \( c \) or \( p \) indicates whether \( K \) is expressed in terms of concentrations or pressures. Equilibrium-constant expressions do not include terms for pure solids or liquids, as their composition does not change throughout the reaction. The standard state of a pure substance is itself, although the quantity may change. The constant value is incorporated into \( K \) and does not need separate accounting.
---
**Deriving Concentrations from Data**
In Part A, equilibrium pressures were provided and could be used directly in the formula for \( K \). However, in Part B, you will be given initial concentrations and only one equilibrium concentration. Use this data to find all three equilibrium concentrations before applying the formula for \( K \).
**Part B**
A reaction was conducted in a sealed vessel at 740°C:
\[ \text{H}_2(g) + \text{I}_2(g) \rightleftharpoons 2\text{HI}(g) \]
* Initial concentrations: \([H_2] = 3.00 \, M\) and \([I_2] = 2.15 \, M\)
* Equilibrium concentration of \([I_2]\) is 0.0700 \( M \).
**Task**
Calculate the equilibrium constant, \( K_c \), for the reaction at this temperature. Provide your answer numerically.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F06ef5119-c7b9-4136-ae1c-12b1af17aba5%2F9a4c22e8-127f-4da6-994a-a0a299387dbb%2Fyugogw_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Understanding Equilibrium Constants**
The equilibrium constant, \( K \), of a reaction at a particular temperature is determined by the concentrations or pressures of the reactants and products at equilibrium.
**For a gaseous reaction with the general form:**
\[ aA + bB \rightleftharpoons cC + dD \]
**The expressions for \( K_c \) and \( K_p \) are:**
\[ K_c = \frac{[C]^c[D]^d}{[A]^a[B]^b} \]
\[ K_p = \frac{(P_C)^c(P_D)^d}{(P_A)^a(P_B)^b} \]
Here, the subscript \( c \) or \( p \) indicates whether \( K \) is expressed in terms of concentrations or pressures. Equilibrium-constant expressions do not include terms for pure solids or liquids, as their composition does not change throughout the reaction. The standard state of a pure substance is itself, although the quantity may change. The constant value is incorporated into \( K \) and does not need separate accounting.
---
**Deriving Concentrations from Data**
In Part A, equilibrium pressures were provided and could be used directly in the formula for \( K \). However, in Part B, you will be given initial concentrations and only one equilibrium concentration. Use this data to find all three equilibrium concentrations before applying the formula for \( K \).
**Part B**
A reaction was conducted in a sealed vessel at 740°C:
\[ \text{H}_2(g) + \text{I}_2(g) \rightleftharpoons 2\text{HI}(g) \]
* Initial concentrations: \([H_2] = 3.00 \, M\) and \([I_2] = 2.15 \, M\)
* Equilibrium concentration of \([I_2]\) is 0.0700 \( M \).
**Task**
Calculate the equilibrium constant, \( K_c \), for the reaction at this temperature. Provide your answer numerically.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY