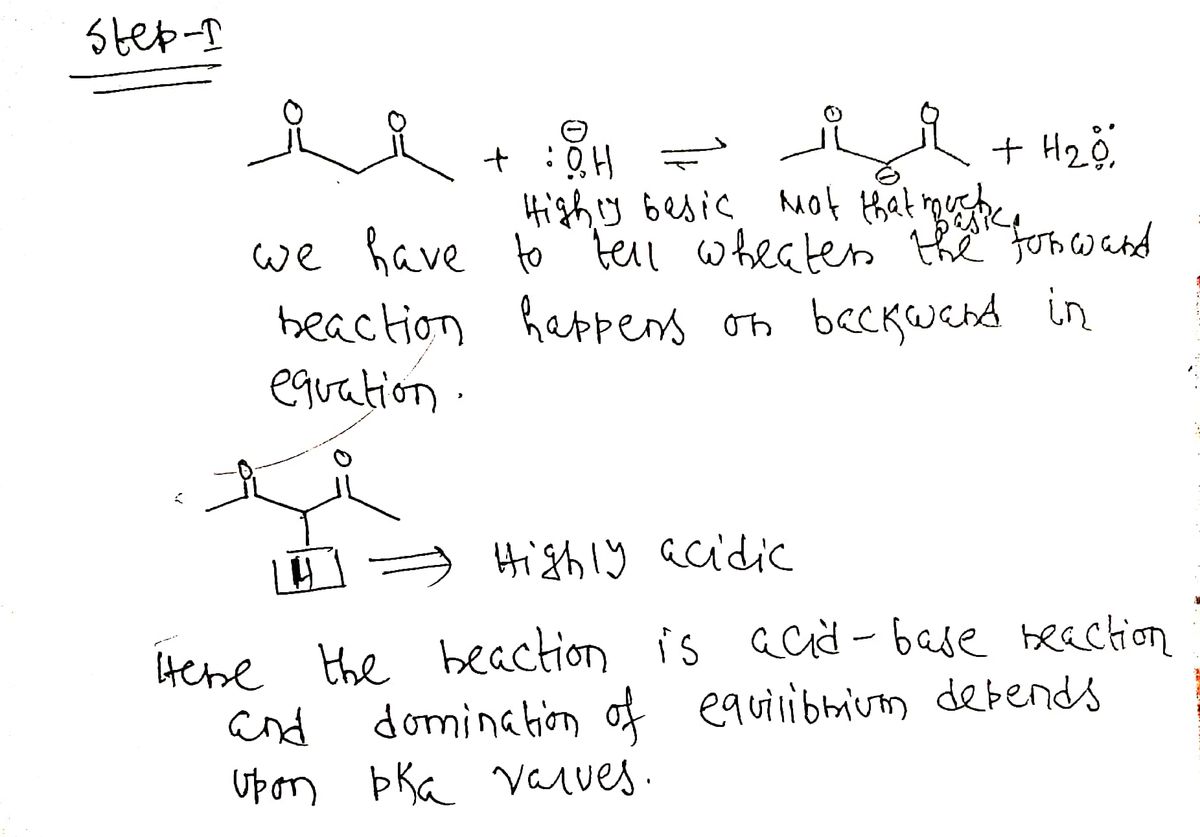
On part c when we identify the equilibrium u guys said it favour the forward direction am confused here , isnt here the less stable the conjugate base it the weaker the acid and here in this part on the CB it showes that the conjugate base with lone pair that means it is less stable and dosent this makes the acid on the reactant weaker and if so since nature favour the weaker shouldn't the equilibrium shift backward? Thank u
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
On part c when we identify the equilibrium u guys said it favour the forward direction am confused here , isnt here the less stable the conjugate base it the weaker the acid and here in this part on the CB it showes that the conjugate base with lone pair that means it is less stable and dosent this makes the acid on the reactant weaker and if so since nature favour the weaker shouldn't the equilibrium shift backward? Thank u
![**Step 2**
(a) Loan pair and electron movement arrows
**[Diagram]**
The diagram shows a molecule with loan pairs and electron movement indicated by curved arrows. An OH- group is presented as reacting with a carbonyl group.
(b) Molecule structure with labels:
- Electrophile
- Nucleophile
**[Diagram]**
The diagram indicates positions of the electrophile and nucleophile within the reaction, showing interaction between a carbonyl group and an OH- group.
(c) Text explanation:
Forward side dominate in equilibrium due to the formation of resonating structure
(d) Resonating structure of product.
**[Diagram]**
This diagram illustrates the resonating structures with double-headed arrows between different forms of the molecule, emphasizing the stability and equilibrium dominance due to resonance.
---
**Feedback Section**
*Was This Helpful?* [Thumbs Up] [Thumbs Down]
**Support Section**
*Still Need Help?*
- *Follow Up Question*
- *Feedback*](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fa6ecc947-ff90-4893-8cf1-1ed20ff242ac%2Fbaeed8fd-e7e7-4ac6-abf0-7782f04d0904%2Fcmpmk97_processed.jpeg&w=3840&q=75)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









