Note these acronyms: HOAC = Acetic Acid; NaOAc = Sodium Acetate; THF = tetrahydrofuran Ph = Phenyl = C6H5; DMSO = Dimethyl Sulfoxide, H3C-O-H = methanol, Zn = Zinc, D = heavy Hydrogen (1 proton and 1 neutron). Acetone = Acetic Acid = THF= Ph= CH3 HO, 1. Write the products of the following reactions. If there is no reaction write NR. For full credit clearly indicate cis or trans relationships in cyclic compounds, where appropriate. ( +к Мпо , кон, cold CHa + KMNO4, KOH, cold
Note these acronyms: HOAC = Acetic Acid; NaOAc = Sodium Acetate; THF = tetrahydrofuran Ph = Phenyl = C6H5; DMSO = Dimethyl Sulfoxide, H3C-O-H = methanol, Zn = Zinc, D = heavy Hydrogen (1 proton and 1 neutron). Acetone = Acetic Acid = THF= Ph= CH3 HO, 1. Write the products of the following reactions. If there is no reaction write NR. For full credit clearly indicate cis or trans relationships in cyclic compounds, where appropriate. ( +к Мпо , кон, cold CHa + KMNO4, KOH, cold
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:### Text and Diagrams for Educational Website
**Acronyms and Compounds:**
- **HOAc**: Acetic Acid
- **NaOAc**: Sodium Acetate
- **THF**: Tetrahydrofuran
- **Ph**: Phenyl = C₆H₅
- **DMSO**: Dimethyl Sulfoxide
- **H₃C-O-H**: Methanol
- **Zn**: Zinc
- **D**: Heavy Hydrogen (1 proton and 1 neutron)
**Structures:**
- **Acetone**:
- Structure: O=C(CH₃)₂
- **Acetic Acid**:
- Structure: O=C(CH₃)OH
- **THF**:
- Structure: Five-membered ring with one oxygen
- **Phenyl (Ph)**:
- Structure: Benzene ring (six-membered with alternating double bonds)
**Chemical Reactions:**
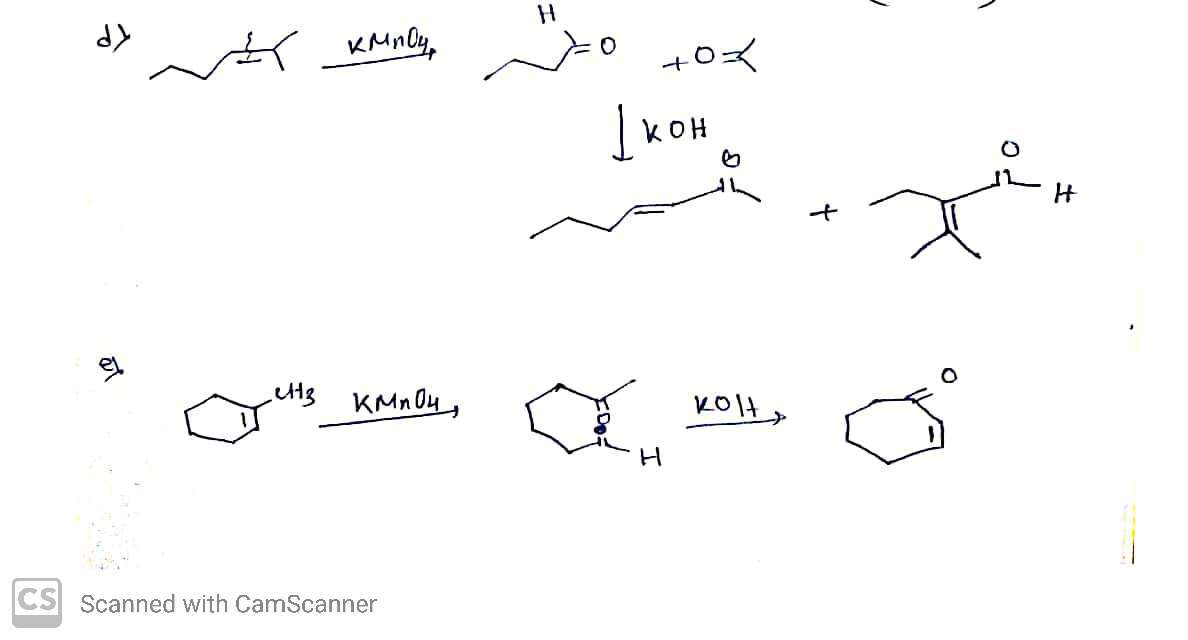
1. **Write the products of the following reactions. If there is no reaction, write NR. Clearly indicate cis or trans relationships in cyclic compounds, where appropriate.**
#### Reactions:
1. **Reaction 1:**
- **Starting Material**:
- Hexene structure with a terminal alkene group.
- **Reagent**:
- KMnO₄, KOH, cold
- **Expected Product**:
- Syn-diol product likely due to cold KMnO₄ addition.
2. **Reaction 2:**
- **Starting Material**:
- Cyclohexene with a methyl group.
- **Reagent**:
- KMnO₄, KOH, cold
- **Expected Product**:
- Syn-diol product on the cyclohexane ring.
3. **Reaction 3:**
- **Starting Material**:
- Heptene structure with an internal alkene.
- **Reagent**:
- H₂, 1 atm, RT, Ni (catalyst)
- **Expected Product**:
- Saturated alkane product through hydrogenation.
4. **Reaction 4:**
- **Starting Material**:
- Cyclohexene with phenyl group attached.
- **Reagent**:
- H₂, 1 atm, RT
Expert Solution
Step 1
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY