Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
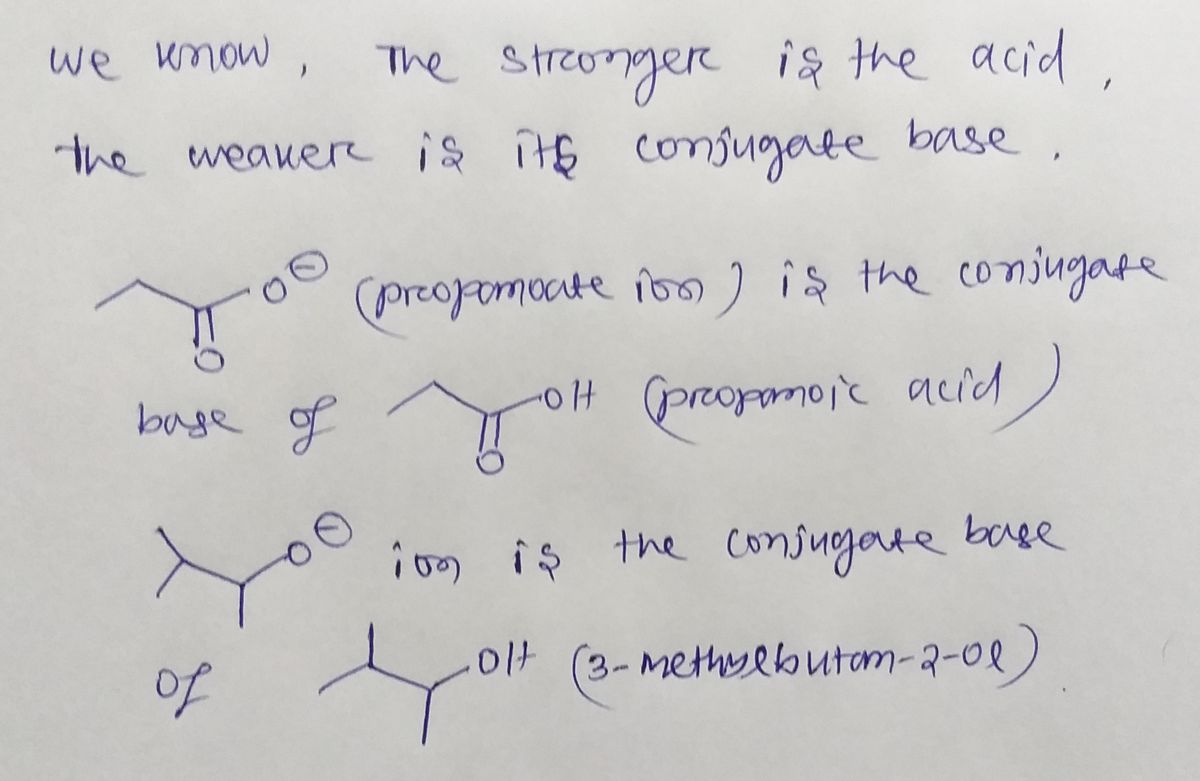
For each set of compounds shown below, circle the stronger base.

Transcribed Image Text:### Understanding Organic Compounds: Structures and Ions
In this image, we see the structural representations of four different organic compounds, each featuring ionic components. These are depicted using line-angle formulas common in organic chemistry.
1. **First Structure:**
- This compound is an acetate ion, characterized by a carbonyl group (a carbon double-bonded to oxygen) adjacent to a negatively charged oxygen ion.
2. **Second Structure:**
- Here, we have an isopropoxide ion, featuring a central carbon atom bonded to two methyl groups and an oxygen ion with a negative charge.
3. **Third Structure:**
- This diagram illustrates a nitrophenolate ion, where a six-membered aromatic ring, indicated by alternating single and double bonds, carries a nitro group (NO₂) and a negatively charged oxygen ion.
4. **Fourth Structure:**
- Similar to the third, this compound is another form of nitrophenolate ion. The nitro group is positioned differently relative to the negatively charged oxygen ion on the ring, demonstrating potential resonance forms.
By examining these structures, we can deduce how organic compounds can vary by altering substitutions and positions of functional groups, affecting their chemical reactivity and properties.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY