Following is an outline of a synthesis of the bronchodilator carbuterol, a beta-2 adrenergic blocker with high selectivity for airway smooth muscle receptors.
Q.Why is it necessary to add the benzyl group, PhCH2—, as a blocking group in Step 1?

Given is an outline of a synthesis of the bronchodilator carbuterol, a beta-2 adrenergic blocker with high selectivity for airway smooth muscle receptors.
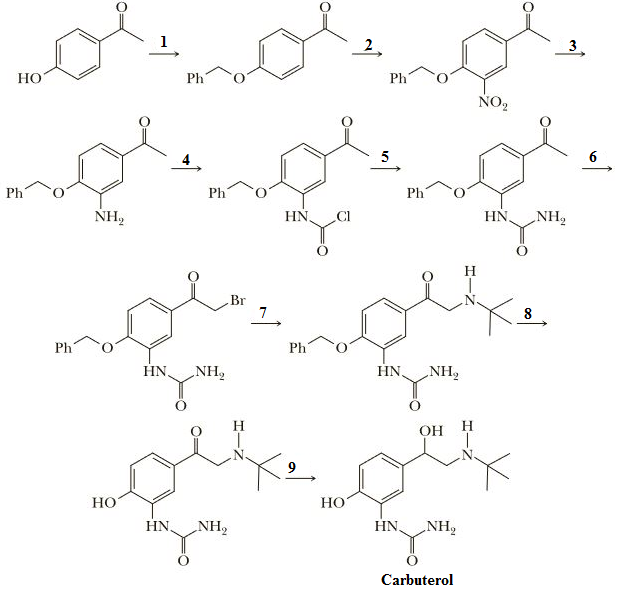
It necessary to add the benzyl group, PhCH2—, as a blocking group in Step 1 has to be explained below,
It is important to create the phenol hydroxyl bunch by utilizing benzyl liquor through ether union on the grounds that, in sync, there is a chance of the development of five-membered lactam, to stay away from these item phenol bunch is to be ensured.
Therefore, the response is given below,
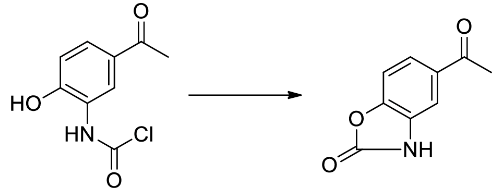
Step by step
Solved in 3 steps with 3 images









