Name the following pairs of compounds according to recommendation of the IUPAC, and state if they are chiral or not. H3C D H3C CH3 CH3 H₂C H3C H CH3 CH3 CH3
Name the following pairs of compounds according to recommendation of the IUPAC, and state if they are chiral or not. H3C D H3C CH3 CH3 H₂C H3C H CH3 CH3 CH3
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Title: Naming and Chirality of Cycloalkane Compounds**
**Objective:**
Learn how to name pairs of cycloalkane compounds according to IUPAC recommendations and determine their chirality.
---
**Compound Analysis:**
1. **First Pair:**
- **Diagram Description:**
- The first structure is a cyclohexane ring with no substituents shown.
- The second structure is a cyclohexane ring with two substituents: two methyl groups (CH₃) at carbon-1 and carbon-4.
- **Chirality:**
- The first structure is achiral (lacks asymmetric carbon centers).
- The second structure is achiral, as it has a plane of symmetry between the substituents.
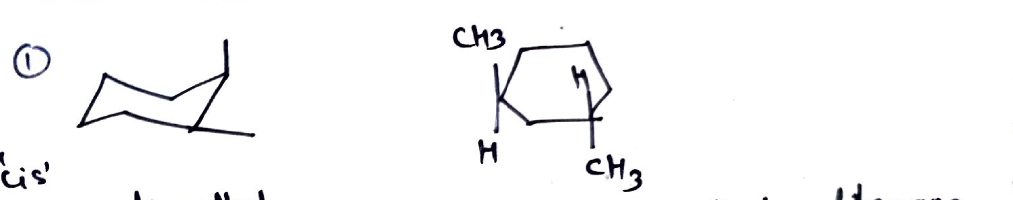
2. **Second Pair:**
- **Diagram Description:**
- The left structure: A cyclohexane ring with methyl groups on carbon-1 and carbon-3, both equatorial.
- The right structure: A cyclohexane ring with a methyl group on carbon-1 equatorial and another on carbon-4 axial.
- **Chirality:**
- Both structures are chiral, due to the lack of a plane of symmetry and presence of distinct spatial arrangement of substituents.
3. **Third Pair:**
- **Diagram Description:**
- The left structure: A cyclohexane ring with a single methyl group at the top, labeled with a wedge to indicate three-dimensional orientation.
- The right structure: A cyclohexane ring with a single methyl group on the right side, labeled with a wedge to indicate three-dimensional orientation.
- **Chirality:**
- Both structures are achiral, as they only contain one substituent and have no stereogenic centers.
---
**Conclusion:**
These examples demonstrate how to identify chiral centers and name cycloalkane derivatives according to IUPAC standards. Identifying chiral centers involves analyzing the three-dimensional arrangement of substituents on the cyclohexane ring.

Transcribed Image Text:The image displays several chemical structures:
1. **Cyclohexane Derivatives**:
- The first structure is a cyclohexane ring with a methyl group (CH₃) attached, indicating a substituted cyclohexane.
- The second structure also features a cyclohexane ring with a methyl group (H₃C) attached, possibly depicting a different stereochemical orientation.
2. **Tert-Butylcyclohexane**:
- Two molecules of cyclohexane are shown with a tert-butyl group (t-Bu) attached. These structures may illustrate different stereochemical conformations or orientations of the tert-butyl group.
3. **Ethanediol**:
- This structure is linear with two hydroxyl groups (-OH) attached to an ethane backbone, showing the compound ethylene glycol.
4. **Cyclopropanediol**:
- A cyclopropane ring with two hydroxyl groups attached, representing a diol variant of cyclopropane.
These diagrams can be used to illustrate various concepts in organic chemistry, such as stereochemistry, functional groups, and the structural diversity of simple organic molecules.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY