MEwncg8 Tof4a5q5fmgWG31RYRVbHwHNgmChwiL Folv0-EaQSZF3)X-ANG UWFa8yspgtJCYxa fegX7x1R-X71oBw7QYibavbS O STATES OF MATTER Calculating the mass of a gas collected over water OOO D Melanie V Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. collected gas Suppose the CO, gas evolved by a certain chemical reaction taking place at 30.0 °C is collected over water, using chemical water reaction an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 26.8 mL. Sketch of a gas-collection apparatus Calculate the mass of CO., that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about alo the reaction conditions and the nature of the gases. 08 Explanation Check 2021 McGraw HE LLC A Rights Reserved T of Use Pvacy Center Accesdly
MEwncg8 Tof4a5q5fmgWG31RYRVbHwHNgmChwiL Folv0-EaQSZF3)X-ANG UWFa8yspgtJCYxa fegX7x1R-X71oBw7QYibavbS O STATES OF MATTER Calculating the mass of a gas collected over water OOO D Melanie V Sometimes in lab we collect the gas formed by a chemical reaction over water (see sketch at right). This makes it easy to isolate and measure the amount of gas produced. collected gas Suppose the CO, gas evolved by a certain chemical reaction taking place at 30.0 °C is collected over water, using chemical water reaction an apparatus something like that in the sketch, and the final volume of gas in the collection tube is measured to be 26.8 mL. Sketch of a gas-collection apparatus Calculate the mass of CO., that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumption about alo the reaction conditions and the nature of the gases. 08 Explanation Check 2021 McGraw HE LLC A Rights Reserved T of Use Pvacy Center Accesdly
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Can you please help with this question I have attached a picture of. Thank you so much!

Transcribed Image Text:**Calculating the Mass of a Gas Collected Over Water**
Sometimes in the lab, we collect the gas formed by a chemical reaction over water. This method makes it easy to isolate and measure the amount of gas produced.
**Example Problem:**
Suppose the CO₂ gas evolved by a certain chemical reaction taking place at 30.0°C is collected over water, using an apparatus similar to the one in the sketch, and the final volume of gas in the collection tube is measured to be 26.8 mL.
**Objective:**
Calculate the mass of CO₂ that is in the collection tube. Round your answer to 2 significant digits. You can make any normal and reasonable assumptions about the reaction conditions and the nature of the gases.
**Sketch Explanation:**
The sketch depicts a typical gas-collection apparatus where:
- Collected gas is shown rising into the gas collection tube from the reaction container.
- The reaction container is connected via a tube where the chemical reaction takes place, producing the gas.
- Water is present in the collection tube, indicating the gas is collected over water.
**Response Box:**
There is a box for inputting the calculated mass of CO₂ in grams. Additionally, there are options to:
- Multiply the answer by 10.
- Clear the response.
- Access further help or information.
**Additional Tools:**
- Explanation button for detailed assistance.
- Check button for submitting and checking the answer.
**Disclaimer:**
© 2021 McGraw Hill LLC. All rights reserved. Terms of Use and Privacy Center are available for reference, alongside accessibility information.
Expert Solution
Step 1
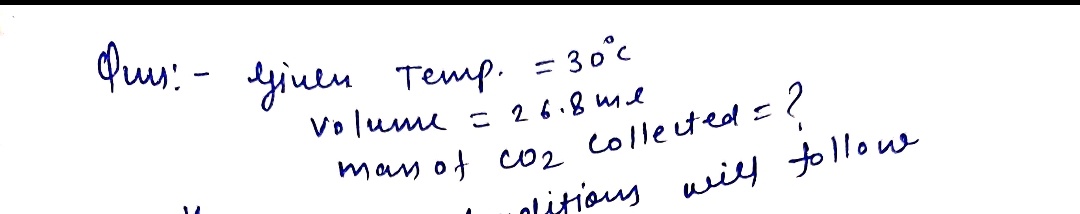
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY