MasteringChemistry: CHE154 S X This Mac can't connect to iCloud b a problem with "ayanaboo10@gma A session.masteringche Open iCloud preferences to fix this probler Later iCloud Prefe
MasteringChemistry: CHE154 S X This Mac can't connect to iCloud b a problem with "ayanaboo10@gma A session.masteringche Open iCloud preferences to fix this probler Later iCloud Prefe
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:MasteringChemistry: CHE154 S X
This Mac can't connect to iCloud b
a problem with "ayanaboo10@gma
A session.masteringche
Open iCloud preferences to fix this probler
Later
iCloud Prefe
<CHE154 S20 Ch17 Sec7-9
Chapter 17 Algorithmic Question 64
Part A
Calculate the pH of a 1.50 M CH3NH3CI solution. K, for methylamine, CH3NH2, is 4.4 x 104.
8.77
1.59
O5.23
12.41
Submit
Request Answer
Provide Feedback
Expert Solution
Step 1
Methylammonium chloride dissociates into quaternary ammonium ion (CH3NH3+) and chloride ions (Cl-). Chloride ion (Cl-) is the conjugate base of a strong acid so it will not influence the pH of the solution. Methylammonium ion is the conjugate acid of a weak base, so an acid-dissociation reaction determines the pH of the solution.
The balance equation is given below,
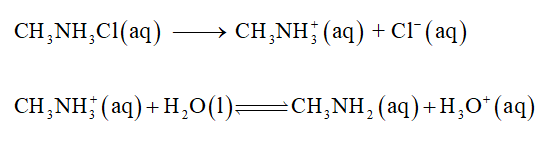
Step 2
Ka value of CH3NH3+ is calculated,
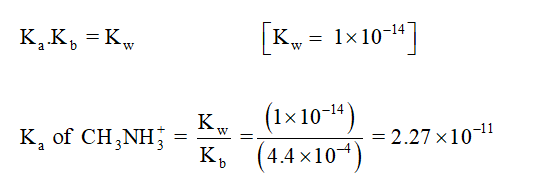
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images






