1- Use the molecular orbital model to predict the bond order and magnetism of each of the following molecules.
a- CO
b- CO+
c- CO2+
(a)
The molecular orbital diagram of CO molecule shows that it contains eight bonding electrons in the σ1, σ3 and pi orbitals and two antibonding electrons in σ2 orbital. It does not contain any unpaired electrons; therefore, it is diamagnetic in nature.
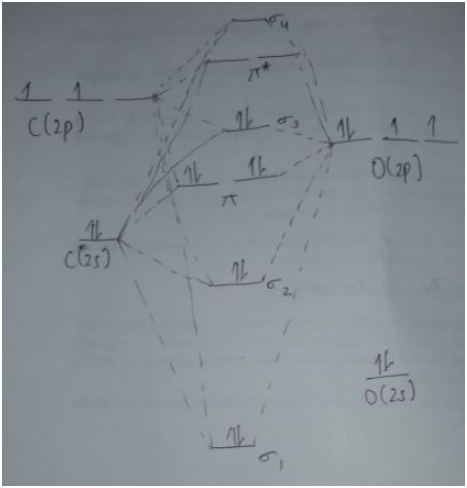
The bond order of CO can be calculated as,
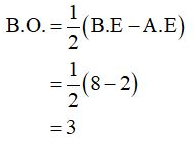
(b)
For the formation of CO+ cation, an electron is removed from the bonding σ3 orbital, therefore, this molecule contains 7 bonding and 2 antibonding electrons. It contains an unpaired electron; therefore, it is paramagnetic in nature.
The bond order of CO+ can be calculated as,
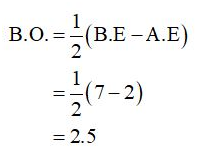
(c)
The molecular orbital diagram of CO2+ molecule shows that it contains eight bonding and seven nonbonding electrons. It contains an unpaired electron; therefore, it is paramagnetic in nature.
Step by step
Solved in 5 steps with 5 images









