In from storm: Q=10x10° L/day An action involves the expansion of how much water a sewer system can handle. Determine the impacted condition of this action during an extreme rainfall Out to a river: _a(t=0)=5x106 L/day Q(t=0)=? L/day Sewer In from houses: Q=30x10° L/day event. There are two flows into the sewer: (1) water +=10° L from households and (2) water from the storm. There are two flows out of the sewer: (3) to a wastewater Out to wastewater plant: Q3=25x106 L/day treatment plant and (4) the rest to a nearby river. Model the sewer water as OD. a) Derive an expression (variables only, no numbers) for the unsteady uniform volume accumulation of water in the sewer (d/dt) in L/day when only 5x106 L/day is initially released to the river. b) Starting from your result to part a, derive an expression for the steady uniform media flowrate Qa(t=). c) Determine the values of d/dt when Q4 =Qa(t=0)= 5x106 L/day and the value of Qa(t=0).
In from storm: Q=10x10° L/day An action involves the expansion of how much water a sewer system can handle. Determine the impacted condition of this action during an extreme rainfall Out to a river: _a(t=0)=5x106 L/day Q(t=0)=? L/day Sewer In from houses: Q=30x10° L/day event. There are two flows into the sewer: (1) water +=10° L from households and (2) water from the storm. There are two flows out of the sewer: (3) to a wastewater Out to wastewater plant: Q3=25x106 L/day treatment plant and (4) the rest to a nearby river. Model the sewer water as OD. a) Derive an expression (variables only, no numbers) for the unsteady uniform volume accumulation of water in the sewer (d/dt) in L/day when only 5x106 L/day is initially released to the river. b) Starting from your result to part a, derive an expression for the steady uniform media flowrate Qa(t=). c) Determine the values of d/dt when Q4 =Qa(t=0)= 5x106 L/day and the value of Qa(t=0).
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%

Transcribed Image Text:In from storm:
Q=10x10° L/day
An action involves the expansion of how much water a
sewer system can handle. Determine the impacted
condition of this action during an extreme rainfall
Out to a river:
_a(t=0)=5x106 L/day
Q(t=0)=? L/day
Sewer
In from houses:
Q=30x10° L/day
event. There are two flows into the sewer: (1) water
+=10° L
from households and (2) water from the storm. There
are two flows out of the sewer: (3) to a wastewater
Out to wastewater plant:
Q3=25x106 L/day
treatment plant and (4) the rest to a nearby river.
Model the sewer water as OD.
a) Derive an expression (variables only, no numbers) for the unsteady uniform volume
accumulation of water in the sewer (d/dt) in L/day when only 5x106 L/day is initially
released to the river.
b) Starting from your result to part a, derive an expression for the steady uniform media
flowrate Qa(t=).
c) Determine the values of d/dt when Q4 =Qa(t=0)= 5x106 L/day and the value of Qa(t=0).
Expert Solution
Step 1
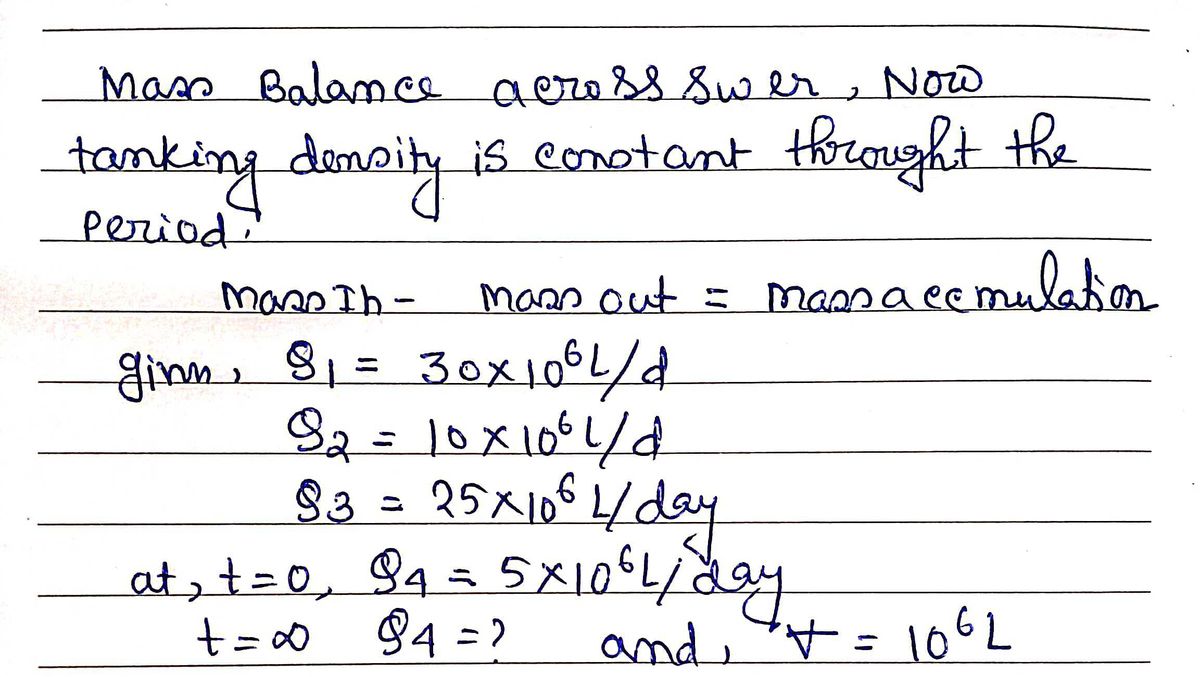
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The