If 100 mls of 0.02 M HCl added to 400 mls of 0.5 M glycine buffer at pH10.4, what is the resultant pH? (pK1 for Gly= 2.2, Pk2=9.4) Part B: What would the pH be if 100 mls of .02 M HCl was added to 400 mls of water?
If 100 mls of 0.02 M HCl added to 400 mls of 0.5 M glycine buffer at pH10.4, what is the resultant pH? (pK1 for Gly= 2.2, Pk2=9.4) Part B: What would the pH be if 100 mls of .02 M HCl was added to 400 mls of water?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section14.8: Acid-base Reactions Of Salts
Problem 14.21CE
Related questions
Question
If 100 mls of 0.02 M HCl added to 400 mls of 0.5 M glycine buffer at pH10.4, what is the resultant pH? (pK1 for Gly= 2.2, Pk2=9.4)
Part B: What would the pH be if 100 mls of .02 M HCl was added to 400 mls of water?
Expert Solution
Step 1
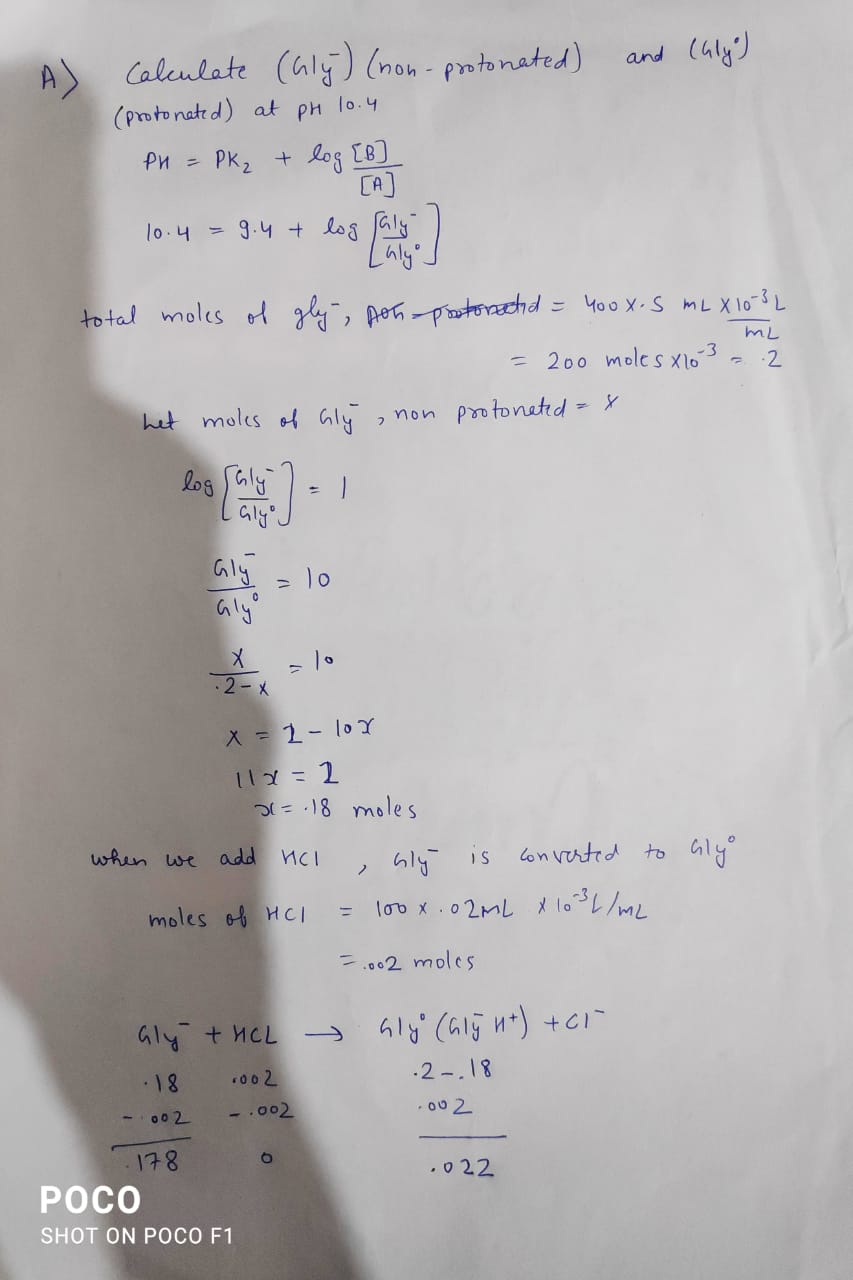
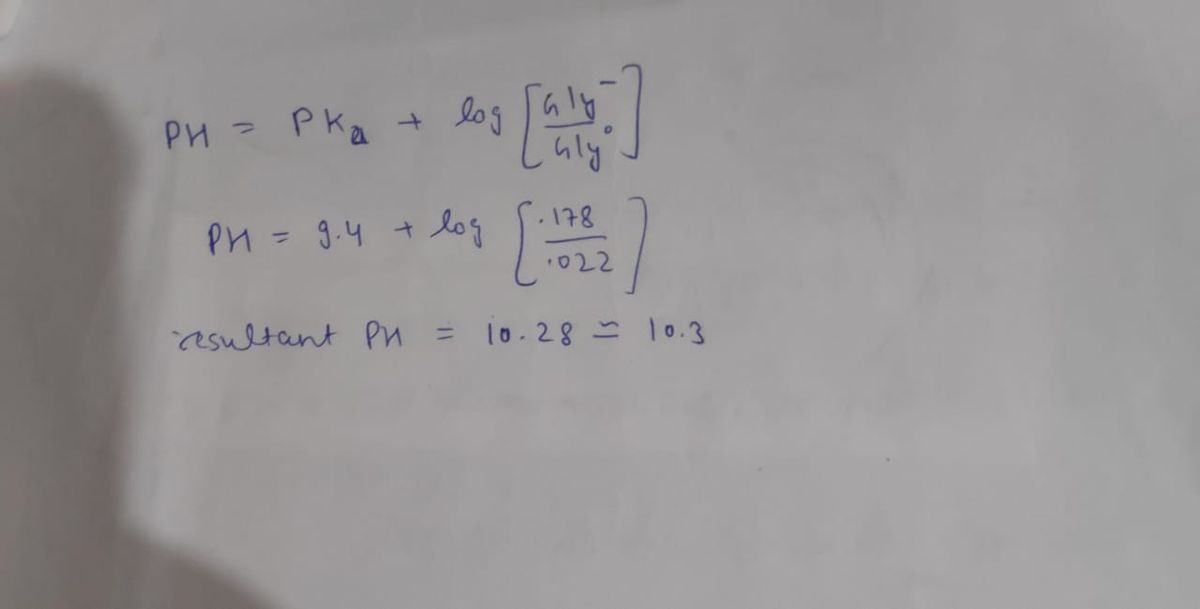
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
