Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:101 Chem101
G CH;NCO - Google Search
X Q Fichas de aprendizaje Chem Ex X +
app.101edu.co
M
Apps
G
M Gmail
YouTube
Maps
а АМAZON
Translate
Gflights
USCIS
Ь ВАТERBLY
C CHEGG > KATAPULK CUBA
SUPERMARKET23
Essay Writing Ser...
G calculator - Googl...
Reading List
Question 8 of 16
Submit
Draw the Lewis structure of CH,NCO (with minimized formal charges) and then
choose the appropriate set of hybridization states for the three central atoms. Your
answer choice is independent of the orientation of your drawn structure.
A) sp3 / sp? / sp
B) sp3 / sp? / sp²
C) sp3 / sp3 / sp³
D) sp / sp? / sp
E) sp³ / sp / sp
Click to edit molecule
+
Expert Solution
Step 1
In Hybridization, atomic orbitals overlaps to form Hybridi orbitals.
In Hybridization sigma bond and lone pair participate.
So, lewis structure of CH3NCO is
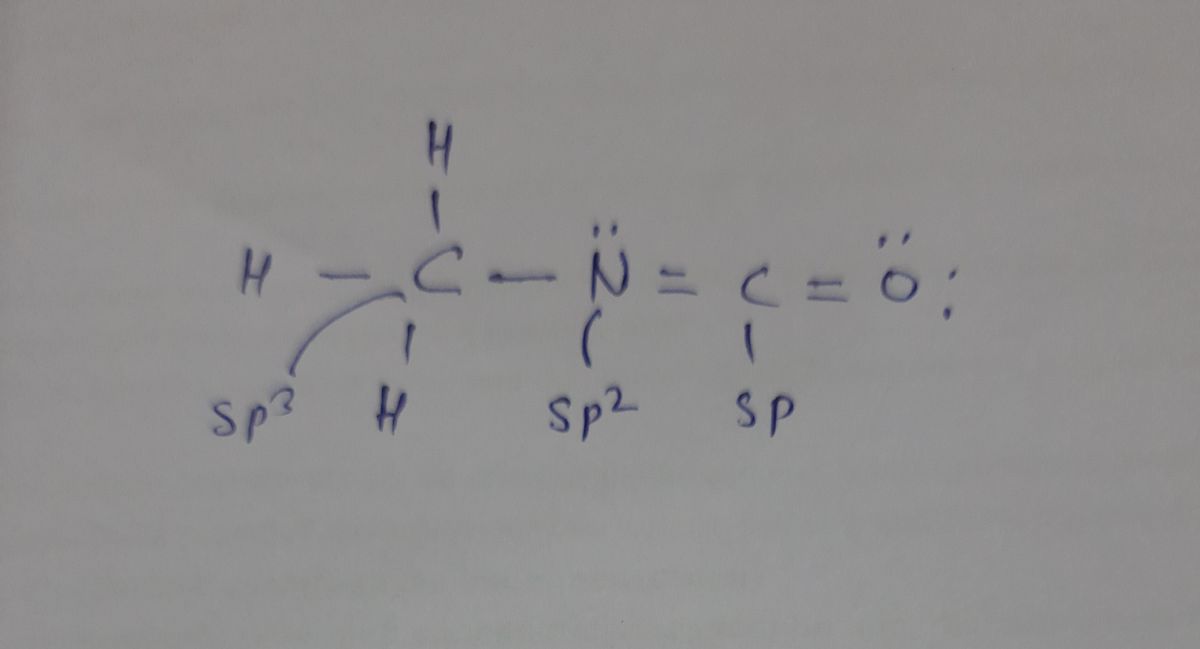
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY