For A 6.5î – 83 + 6k and B = -7î + 5.73 %3D 6k then what is the z component of A x B?
Q: An electron occupying the n = 6 shell of an atom carries z-component orbital angular momentum =…
A: Introduction:- The shell has no atom that has angular momentum..Given:- Objective:-Max. possible…
Q: Explicit expressions for hydrogenic orbitals are given in Tables 7F.1 (for the angular component)…
A:
Q: 1. (a) Use the equation for energy Eigen values that result from the solution to the infinite square…
A: For infinite square well the energy associated with nth quantum number is given by: En=n2h28mL2…
Q: Show that a variation theory treatment of H using trial function = e^-kr as an unnormalized trial…
A:
Q: Calculate the energy difference between the ms = 1/ 2 (“spin up”) and ms = - 1 /2 (“spin down”)…
A: Given data: The magnetic field in the negative z-direction is B=-1.45 T. The expression for the…
Q: A hydrogen atom undergoes a transition from a 2p state to the 1s ground state. In the absence of a…
A:
Q: List all possible sets of quantum numbers (n,l,m1,ms) for the n=3 shell, and determine the number of…
A: Given:- quantum numbers (n,l,m1,ms) for the n=3 shell are following:
Q: 11:29 Fri 1 Dec x no 00 00 CW1 week9-2023 ✓ Assignment 2 4 week9-2023 abc ~~~~~. QUESTION 2:…
A:
Q: The radial part of the Schrödinger equation for the hydrogen atom Ze² ħ² d ƏR (r) ħ² l ( l + 1) 2pr²…
A: Given that The radial part of the Schrödinger equation for the hydrogen atom can be written in the…
Q: The radial wave function for the Sf orbital can be expressed as: Rn, Ar) = Ne-/5 p3 (8-2) where N is…
A: orbitals can be written as l=0, s-orbital l=1, p-orbital l=2,d-orbital l=3,f-orbital
Q: Determine the integral P(r) dr for the radial probability density for the ground state of the…
A:
Q: (a) The L→ K transition of an X-ray tube containing a molybdenum (Z = 42) target occurs at a…
A:
Q: Show that an energy difference of 2 x 10-3 eV for the 3p subshell of sodium accounts for the 0.6-nm…
A: Given: The energy difference is ∆E=2×10-3 eV. The wavelength of the spectral line is λ=589.3 nm.…
Q: Consider the normal Zeeman effect applied to the 3d to 2p transition. (a) Sketch an energy-level…
A: Consider the normal Zeeman effect applied to the 3d to 2p transition.3d -------> 2pTransition…
Q: 1 – 2i An electron is in the spin state x = A 2 Find the expectation value of Sy for an electron in…
A:
Q: If elements beyond Z = 120 are ever synthesized, electrons in these heavy atoms will begin filling a…
A: Given: The atomic number is 120.
Q: How many subshells are in the n=3 shell? Identify each subshell, calculate the maximum number of…
A: Given data The number of shells is n=3 The number of subshells is, l=0 to n-1=0 to 3-1=0,1,2 Thus,…
Q: An electron is in a 3p state in the hydrogen atom, given that the expectation value is 12.5a_0 What…
A: The objective of this question is to find the probability of finding an electron in a specific range…

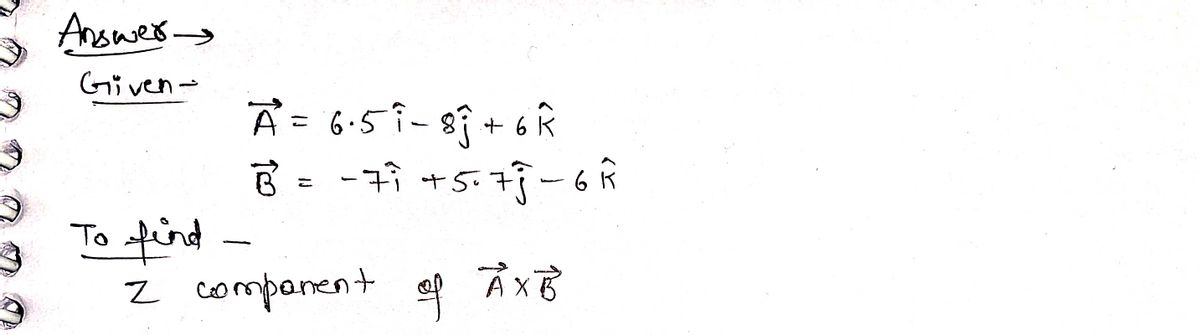
Step by step
Solved in 2 steps with 2 images

- Consider an angular momentum 1 system, represented by the state vector 1 1 2 V26 -3 What is the probability that a measurement of L yields the value 0?(a) What is the magnitude of the orbital angular momentum in a state with e = 2? (b) What is the magnitude of its largest projection on an imposed axis? (a) Number 2.50998008 Units J.s (b) Number 2.11 Units J.sList all the possible quantum numbers (n,l,me) for the n = 5 level in atomic hydrogen.
- Suppose you measure the angular momentum in the z-direction L, for an /= 2 hydrogen atom in the state | > 2 > |0 > +i/ |2 >. The eigenvalues of %3D V10 10 Lz are – 2h, -ħ, 0, ħ, 2ħfor the eigenvectors | – 2 >, |– 1>, |0 >, |1 >, |2 >, respectively. What is AL,? V31 10 7 19 25What wavelengths for the Lα lines did Moseley predict for the missing Z= 43, 61, and 75 elements?