Draw the structure of the product of the reaction between the compound shown below and BF3. CH3CH₂OH • You do not have to consider stereochemistry. You do not have explicitly draw H atoms. • If the reaction involves proton transfer, draw the products in separate sketchers. • If the reaction is a Lewis acid-base reaction, draw the product in one sketcher only. 7-8 3 9.85 OOO. SIF Base + Acid H₂COH изсизон ChemDoodle remove 226 22 4 ·000- F ChemDoodle Conj. base Com). *182 GRA 0. 000. SIF H₂co ChemDoodle Conjacid ✔399-8 H▾ 000 ChemDoodleⓇ
Draw the structure of the product of the reaction between the compound shown below and BF3. CH3CH₂OH • You do not have to consider stereochemistry. You do not have explicitly draw H atoms. • If the reaction involves proton transfer, draw the products in separate sketchers. • If the reaction is a Lewis acid-base reaction, draw the product in one sketcher only. 7-8 3 9.85 OOO. SIF Base + Acid H₂COH изсизон ChemDoodle remove 226 22 4 ·000- F ChemDoodle Conj. base Com). *182 GRA 0. 000. SIF H₂co ChemDoodle Conjacid ✔399-8 H▾ 000 ChemDoodleⓇ
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Is this correct? If not, can you please explain how to do this problem? Thank you!
![### Understanding Acid-Base Reactions: A Detailed Example
**Problem Statement**
Draw the structure of the product of the reaction between the compound shown below and \( BF_3 \).
**Given Compound:**
\[ CH_3CH_2OH \]
### Guidelines
- You do not have to consider stereochemistry.
- You do not have to explicitly draw H atoms.
- If the reaction involves proton transfer, draw the products in separate sketchers.
- If the reaction is a Lewis acid-base reaction, draw the product in one sketcher only.
### Reaction Breakdown
1. **Identifying the Acid and Base**
The first image shows the given compound \( CH_3CH_2OH \) (ethanol) labeled as "Acid" and the Lewis structure of \( BF_3 \) labeled as "Base".
**Ethanol (Acid)**:
\[
H_3C-CH_2-OH
\]
**Boron Trifluoride (Base)**:
\[
BF_3 \quad (\text{with Lewis structure})
\]
The \( BF_3 \) molecule can accept an electron pair, acting as a Lewis acid in this context since it has an empty p-orbital.
2. **Reaction and Products**
The second and third images depict the products of the reaction, labeled "Conjugate Base" and "Conjugate Acid".
**Conjugate Base**:
The ethanol after losing a proton (H+):
\[
H_3C-CH_2-O^-
\]
**Conjugate Acid**:
The \( BF_3 \) after bonding with the hydroxyl group:
\[
H \quad (\text{attached to} \quad BF_3)
\]
Unfortunately, the specific placement of \( BF_3 \) is not clearly shown in the annotation.
### Final Visualization
The products should be visualized as individual entities:
1. **Ethanol Acid Product:**
\[
CH_3CH_2O^- \quad (\text{Conjugate Base})
\]
2. **BF3-based Product:**
\[
BF_3H \quad (\text{Conjugate Acid, specifically where the \( BF_3 \) attaches to an oxygen site})
\]
This visualization helps in understanding the](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3b49abb7-22b1-43f4-8ea9-ededd2a6e7a5%2Fa123fd40-71d7-4a7f-ad03-c1fed010ac45%2F2e3hfg8_processed.png&w=3840&q=75)
Transcribed Image Text:### Understanding Acid-Base Reactions: A Detailed Example
**Problem Statement**
Draw the structure of the product of the reaction between the compound shown below and \( BF_3 \).
**Given Compound:**
\[ CH_3CH_2OH \]
### Guidelines
- You do not have to consider stereochemistry.
- You do not have to explicitly draw H atoms.
- If the reaction involves proton transfer, draw the products in separate sketchers.
- If the reaction is a Lewis acid-base reaction, draw the product in one sketcher only.
### Reaction Breakdown
1. **Identifying the Acid and Base**
The first image shows the given compound \( CH_3CH_2OH \) (ethanol) labeled as "Acid" and the Lewis structure of \( BF_3 \) labeled as "Base".
**Ethanol (Acid)**:
\[
H_3C-CH_2-OH
\]
**Boron Trifluoride (Base)**:
\[
BF_3 \quad (\text{with Lewis structure})
\]
The \( BF_3 \) molecule can accept an electron pair, acting as a Lewis acid in this context since it has an empty p-orbital.
2. **Reaction and Products**
The second and third images depict the products of the reaction, labeled "Conjugate Base" and "Conjugate Acid".
**Conjugate Base**:
The ethanol after losing a proton (H+):
\[
H_3C-CH_2-O^-
\]
**Conjugate Acid**:
The \( BF_3 \) after bonding with the hydroxyl group:
\[
H \quad (\text{attached to} \quad BF_3)
\]
Unfortunately, the specific placement of \( BF_3 \) is not clearly shown in the annotation.
### Final Visualization
The products should be visualized as individual entities:
1. **Ethanol Acid Product:**
\[
CH_3CH_2O^- \quad (\text{Conjugate Base})
\]
2. **BF3-based Product:**
\[
BF_3H \quad (\text{Conjugate Acid, specifically where the \( BF_3 \) attaches to an oxygen site})
\]
This visualization helps in understanding the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
So, in this lewis acid-base reaction, do we need to draw lone pairs or not? Why or why not? Thank you.
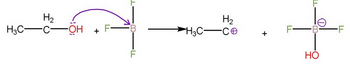
Transcribed Image Text:H₂
H3C-
-OH F
+
LL
F
LL
H3C-
H₂
-CO
F-B
HO
LL
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY