Consider the following reaction: 2N₂O(g) → 2N2(g) + O2(g) ▾ Part A Express the rate of the reaction in terms of the change in concentration of each of the reactants and products. 1 A/N₂0] Rate At O Rate = Ⓒ Rate = ✓ Correct Part B = =- At 1 AN₂ Rate = -10 = N -- At At Submit Previous Answers Rate = Submit Part C A[N₂0 At AIN₂0] At AN₂0] At Submit =11202 N140, == 1 A[N₂] 2 At In the first 13.0s of the reaction, 1.9x10-2 mol of O₂ is produced in a reaction vessel with a volume of 0.280 L. What is the average rate of the reaction over this time interval? Express your answer using two significant figures. IVE] ΑΣΦ Request Answer A(O₂) At = Request Answer At 14/01 At A[0₂] At Predict the rate of change in the concentration of N₂O over this time interval. In other words, what is A[N₂O)/At? Express your answer using two significant figures. 195] ΑΣΦΑ ? → C M.s1 ? M-81
Consider the following reaction: 2N₂O(g) → 2N2(g) + O2(g) ▾ Part A Express the rate of the reaction in terms of the change in concentration of each of the reactants and products. 1 A/N₂0] Rate At O Rate = Ⓒ Rate = ✓ Correct Part B = =- At 1 AN₂ Rate = -10 = N -- At At Submit Previous Answers Rate = Submit Part C A[N₂0 At AIN₂0] At AN₂0] At Submit =11202 N140, == 1 A[N₂] 2 At In the first 13.0s of the reaction, 1.9x10-2 mol of O₂ is produced in a reaction vessel with a volume of 0.280 L. What is the average rate of the reaction over this time interval? Express your answer using two significant figures. IVE] ΑΣΦ Request Answer A(O₂) At = Request Answer At 14/01 At A[0₂] At Predict the rate of change in the concentration of N₂O over this time interval. In other words, what is A[N₂O)/At? Express your answer using two significant figures. 195] ΑΣΦΑ ? → C M.s1 ? M-81
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.36PAE: The reaction NO(g) + O,(g) — NO,(g) + 0(g) plays a role in the formation of nitrogen dioxide in...
Related questions
Question
![Consider the following reaction:
2N₂O(g) → 2N2(g) + O2(g)
Part A
Express the rate of the reaction in terms of the change in concentration of each of the reactants and products.
Rate = 1 A[₂0]
At
O Rate =
O Rate -
Submit
Rate = -A[₂0]
At
Correct
Part B
Rate =
Submit
A[N₂O]
At
Part C
A[N₂0]
At
A[N₂O]
At
Previous Answers
Submit
Request Answer
1 A[N₂]
2
At
At
In the first 13.0 s of the reaction, 1.9x10-2 mol of O₂ is produced in a reaction vessel with a volume of 0.280 L. What is the average rate of the reaction over this time interval?
Express your answer using two significant figures.
IVE| ΑΣΦ
Request Answer
A[N₂]
At
1 A[N₂]
At
1/1
A[0₂]
At
A[0₂]
At
A[0₂]
A[0₂]
At
Predict the rate of change in the concentration of N₂O over this time interval. In other words, what is A[N₂O]/At?
Express your answer using two significant figures.
IVE| ΑΣΦ
?
M-s-1
?
M-s-1](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F3caf0467-ca0e-4770-b0d8-25bdaac915d0%2Fa340c6de-abe0-4016-8af9-2cc1110bc194%2Fp7zis_processed.png&w=3840&q=75)
Transcribed Image Text:Consider the following reaction:
2N₂O(g) → 2N2(g) + O2(g)
Part A
Express the rate of the reaction in terms of the change in concentration of each of the reactants and products.
Rate = 1 A[₂0]
At
O Rate =
O Rate -
Submit
Rate = -A[₂0]
At
Correct
Part B
Rate =
Submit
A[N₂O]
At
Part C
A[N₂0]
At
A[N₂O]
At
Previous Answers
Submit
Request Answer
1 A[N₂]
2
At
At
In the first 13.0 s of the reaction, 1.9x10-2 mol of O₂ is produced in a reaction vessel with a volume of 0.280 L. What is the average rate of the reaction over this time interval?
Express your answer using two significant figures.
IVE| ΑΣΦ
Request Answer
A[N₂]
At
1 A[N₂]
At
1/1
A[0₂]
At
A[0₂]
At
A[0₂]
A[0₂]
At
Predict the rate of change in the concentration of N₂O over this time interval. In other words, what is A[N₂O]/At?
Express your answer using two significant figures.
IVE| ΑΣΦ
?
M-s-1
?
M-s-1
Expert Solution
Step 1
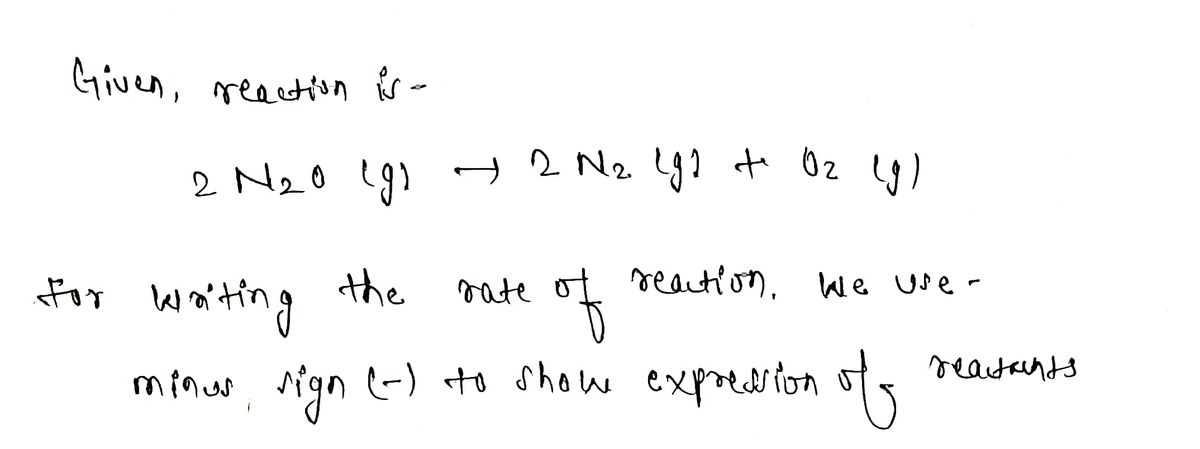
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning