Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 3.01 × 103 at 900 K. If an initial mixture of 0.0216 mol SO2 and 0.0148 mol O2 reacts in a 1.0 L vessel, what will be the concentration of SO3 at equilibrium? 2 SO2(g) + O2(g) =2 SO3(g) 1 2 3 NEXT へ Based on the given values, set up ICE table in order to determine the unknown. 2 SO2(g) O2(g) 2 SO3(g) + Initial (M) 0.0216 0.0148 Change (M) -2x +2x -- Equilibrium (M) 0.0216 - x 0.0148 - x +2x
Consider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 3.01 × 103 at 900 K. If an initial mixture of 0.0216 mol SO2 and 0.0148 mol O2 reacts in a 1.0 L vessel, what will be the concentration of SO3 at equilibrium? 2 SO2(g) + O2(g) =2 SO3(g) 1 2 3 NEXT へ Based on the given values, set up ICE table in order to determine the unknown. 2 SO2(g) O2(g) 2 SO3(g) + Initial (M) 0.0216 0.0148 Change (M) -2x +2x -- Equilibrium (M) 0.0216 - x 0.0148 - x +2x
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:Consider the equilibrium system described by the chemical reaction below, which
has a value of Kc equal to 3.01 x 103 at 900 K. If an initial mixture of 0.0216 mol
SO2 and 0.0148 mol O2 reacts in a 1.0 L vessel, what will be the concentration of
SO3 at equilibrium?
2 SO2(g) + O2(g) = 2 SO3(g)
1
2
3
NEXT
>
Based on the given values, set up ICE table in order to determine the unknown.
2 SO2(g)
O2(g)
2 SO3(g)
+
Initial (M)
0.0216
0.0148
Change (M)
-2x
+2x
-X
Equilibrium (M)
0.0216 - x
0.0148 - x
+2x
1L
Expert Solution
Step 1
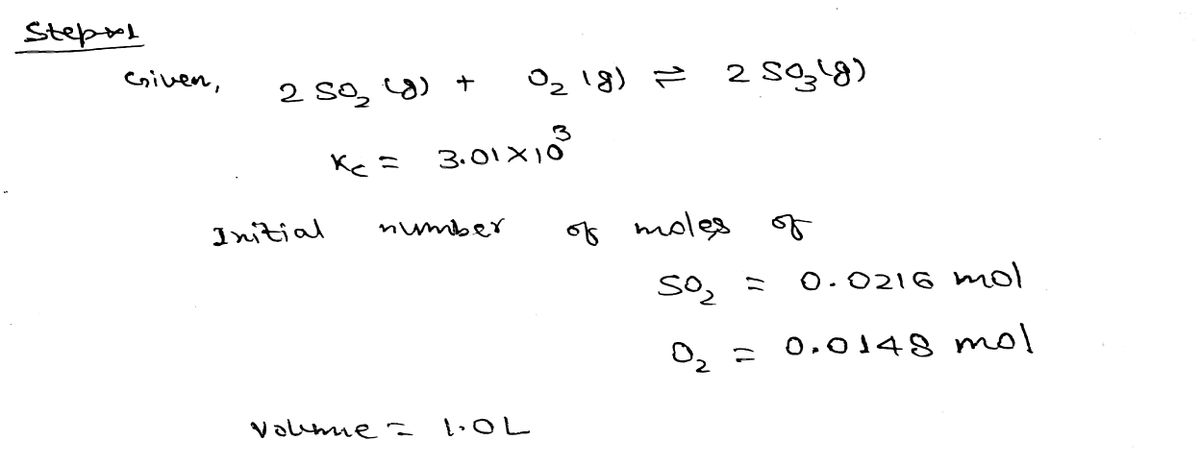
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY