Component Start Total Bottom Phase Top Phase PEG (1) Dextran (2) Water 6.50 3.28 15.83 80.89 8.82 3.70 8.80 84.70 87.48 In Kp, = In = A;(o - o)
A phase-distribution measurement was made for the PEG 3400–dextran T40–water system at 4oC. The following results give the starting composition and the equilibrium compositions of the two resulting phases, all in wt%.Use the Diamond–Hsu method of (8-93) to compute the values of Ai for dextran and PEG. Use those values to produce a tie line with one end at 11.19 wt% PEG in the top phase.


A phase distribution measurement was made for the PEG 3400-dextran T-40 water system at 40C
The composition of the PEG, dextran and water in the top and bottom phases is given in the table.
The diamond-Hsu method is used for measurement of the phase distribution.
According to the Diamond-Hsu the relation to calculate the empirical parameter is,
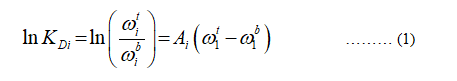
Where,
KDi represents the partition coefficient.
Ai represents the empirical parameter.
ω represents the composition in weight fraction.
t represents the composition of top phase.
b represents the composition of bottom phase.
The empirical parameter for PEG is calculated as follows:
Since the data given in the weight fraction not in distribution coefficient, the equation (1) becomes,
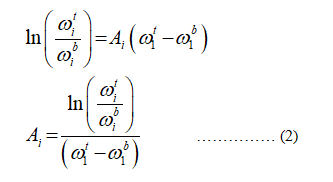
From the data table given in the question,
The composition of PEG in top and bottom phase is given as:
ωtPEG = 0.0882
ωbPEG = 0.0328
Plugin the values in equation (2)
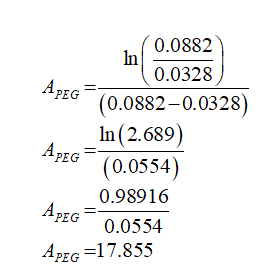
The empirical coefficient for dextran is calculated.
The formula to calculate the empirical coefficient becomes,
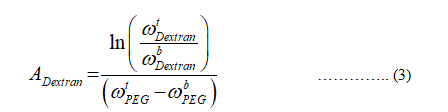
From the data table given in the table, the composition of dextran in the top and bottom phase is given as:
ωtPEG = 0.0882
ωbPEG = 0.0328
ωtDextran = 0.0370
ωbDextran = 0.1583
Plugin the values in equation (2)

Step by step
Solved in 6 steps with 12 images









