Complete the blanks in each column, as in the first example: Name of the Magnesium Lithium ion Sulfide ion particle atom charge 3+ condensed e [He]3s? [He]2s?2p? [Kr]5s?4d10 configuration atomic Lewis • Mg. :Cl: structure
Complete the blanks in each column, as in the first example: Name of the Magnesium Lithium ion Sulfide ion particle atom charge 3+ condensed e [He]3s? [He]2s?2p? [Kr]5s?4d10 configuration atomic Lewis • Mg. :Cl: structure
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
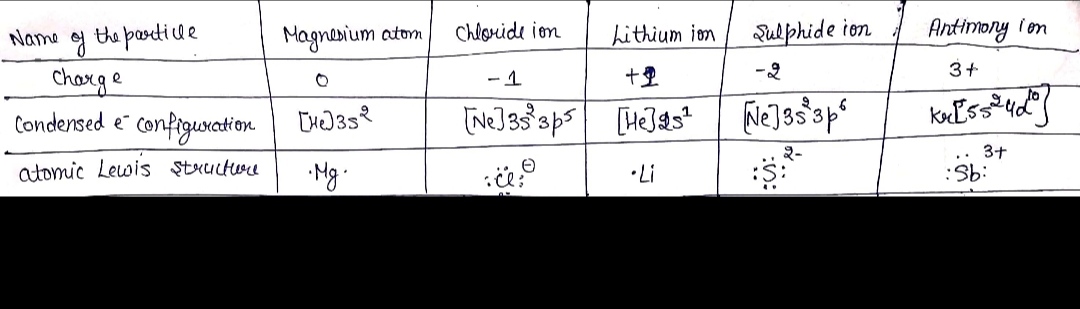
![**Table: Completing the Blanks**
| Name of the particle | Magnesium atom | Lithium ion | Sulfide ion |
|----------------------|----------------|-------------|-------------|
| charge | 0 | 0 | 3+ |
| condensed e⁻ configuration | [He]3s² | [He]2s²2p² | [Kr]5s²4d¹⁰ |
| atomic Lewis structure | ·Mg· | | |
**Instructions:**
Complete the blanks in each column, as in the first example.
**Explanation:**
- **Name of the Particle**: Lists the type of atom or ion.
- **Charge**: Indicates the net electric charge. In this example, the charges are 0 for both Magnesium atom and Lithium ion, and 3+ for Sulfide ion.
- **Condensed Electron Configuration**: Uses the noble gas shorthand notation to represent electron arrangements. Magnesium is shown as [He]3s².
- **Atomic Lewis Structure**: Represents the valence electrons of an element as dots around the chemical symbol. Magnesium is represented as ·Mg· with dots on each side.
Fill in the appropriate electron configuration and Lewis structures for the Lithium ion and Sulfide ion as part of the exercise.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F7e6fe4d9-c522-461f-bc62-94d23fd62501%2Fe33eef42-2220-40d2-af12-249e4c98ac48%2Fb8ogckf_processed.png&w=3840&q=75)
Transcribed Image Text:**Table: Completing the Blanks**
| Name of the particle | Magnesium atom | Lithium ion | Sulfide ion |
|----------------------|----------------|-------------|-------------|
| charge | 0 | 0 | 3+ |
| condensed e⁻ configuration | [He]3s² | [He]2s²2p² | [Kr]5s²4d¹⁰ |
| atomic Lewis structure | ·Mg· | | |
**Instructions:**
Complete the blanks in each column, as in the first example.
**Explanation:**
- **Name of the Particle**: Lists the type of atom or ion.
- **Charge**: Indicates the net electric charge. In this example, the charges are 0 for both Magnesium atom and Lithium ion, and 3+ for Sulfide ion.
- **Condensed Electron Configuration**: Uses the noble gas shorthand notation to represent electron arrangements. Magnesium is shown as [He]3s².
- **Atomic Lewis Structure**: Represents the valence electrons of an element as dots around the chemical symbol. Magnesium is represented as ·Mg· with dots on each side.
Fill in the appropriate electron configuration and Lewis structures for the Lithium ion and Sulfide ion as part of the exercise.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY