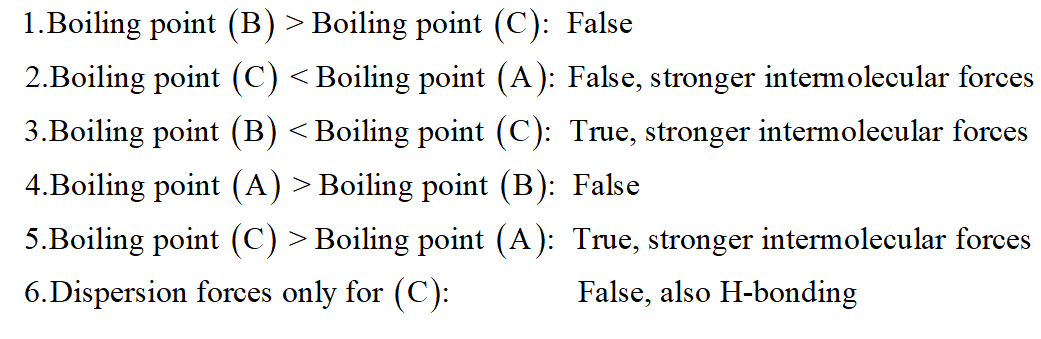
Comparing the boiling points of:A) C3H8B) C5H12C) C2H5OHand identify the following statements as either True or False: True / False: Boiling point (B) > Boiling point (C) True / False: Boiling point (C) < Boiling point (A) True / False: Boiling point (B) < Boiling point (C) True / False: Boiling point (A) > Boiling point (B) True / False: Boiling point (C) > Boiling point (A) True / False: Dispersion forces only for (C) True / False: (B) exhibits hydrogen bonding True / False: (A) exhibits hydrogen bonding True / False: Dispersion forces only for (B) True / False: (C) exhibits hydrogen bonding True / False: Dispersion forces only for (A) True / False: Boiling point (A) < Boiling point (B)
Comparing the boiling points of:
A) C3H8
B) C5H12
C) C2H5OH
and identify the following statements as either True or False:
True / False: Boiling point (B) > Boiling point (C)
True / False: Boiling point (C) < Boiling point (A)
True / False: Boiling point (B) < Boiling point (C)
True / False: Boiling point (A) > Boiling point (B)
True / False: Boiling point (C) > Boiling point (A)
True / False: Dispersion forces only for (C)
True / False: (B) exhibits hydrogen bonding
True / False: (A) exhibits hydrogen bonding
True / False: Dispersion forces only for (B)
True / False: (C) exhibits hydrogen bonding
True / False: Dispersion forces only for (A)
True / False: Boiling point (A) < Boiling point (B)
Boiling points:
Step by step
Solved in 2 steps with 2 images









