Choose a chemical reaction that is used in industry, research, or nature. DO NOT USE the photosynthesis process or the cellular respiration process. Photosynthesis and cellular respiration are not single chemical reactions; they are each a series of chemical reactions. For the reaction that you choose: 1) Write the balanced chemical equation for the reaction (include the phases). 2) What is/are the reactant(s)? 3) What is/are the product(s)? 4) Why is this reaction important in either industry, research, or nature?
Choose a chemical reaction that is used in industry, research, or nature. DO NOT USE the photosynthesis process or the cellular respiration process. Photosynthesis and cellular respiration are not single chemical reactions; they are each a series of chemical reactions. For the reaction that you choose: 1) Write the balanced chemical equation for the reaction (include the phases). 2) What is/are the reactant(s)? 3) What is/are the product(s)? 4) Why is this reaction important in either industry, research, or nature?
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter8: Reactions In Aqueous Solutions
Section8.3: Classifying Reactions
Problem 5RQ
Related questions
Question
Choose a chemical reaction that is used in industry, research, or nature. DO NOT USE the photosynthesis process or the cellular respiration process. Photosynthesis and cellular respiration are not single chemical reactions ; they are each a series of chemical reactions.
For the reaction that you choose:
1) Write the balanced chemical equation for the reaction (include the phases).
2) What is/are the reactant(s)?
3) What is/are the product(s)?
4) Why is this reaction important in either industry, research, or nature?

Transcribed Image Text:The image illustrates four different types of chemical reactions using a visual representation of molecules composed of colored spheres labeled with letters.
1. **Synthesis Reaction:**
- Left Side: A single blue sphere labeled "A" and a single red sphere labeled "B" are shown separately with a plus sign between them.
- Right Side: The spheres "A" and "B" are shown connected together.
- Description: This diagram represents a synthesis reaction where two or more simple substances combine to form a more complex compound (A + B → AB).
2. **Decomposition Reaction:**
- Left Side: A molecule composed of a blue sphere "A" and a red sphere "B" connected together.
- Right Side: The spheres "A" and "B" are separate with a plus sign between them.
- Description: This diagram shows a decomposition reaction where a complex molecule breaks down into simpler parts (AB → A + B).
3. **Single Replacement Reaction:**
- Left Side: A molecule with a blue sphere "A" and red sphere "B" connected, along with a separate green sphere "C".
- Right Side: The blue sphere "A" is now connected to the green sphere "C" with the red sphere "B" shown separately.
- Description: This diagram illustrates a single replacement reaction where one element replaces another in a compound (AB + C → AC + B).
4. **Double Replacement Reaction:**
- Left Side: Two molecules: one with a blue sphere "A" and red sphere "B" connected, and another with green sphere "C" and yellow sphere "D" connected.
- Right Side: The blue sphere "A" is now connected to the yellow sphere "D", and the green sphere "C" is connected to the red sphere "B".
- Description: This diagram depicts a double replacement reaction in which components of two compounds exchange places to form two new compounds (AB + CD → AD + CB).
These illustrations provide a clear visual representation of basic chemical reactions, helping to understand how atoms or groups of atoms rearrange during chemical processes.
Expert Solution
Step 1
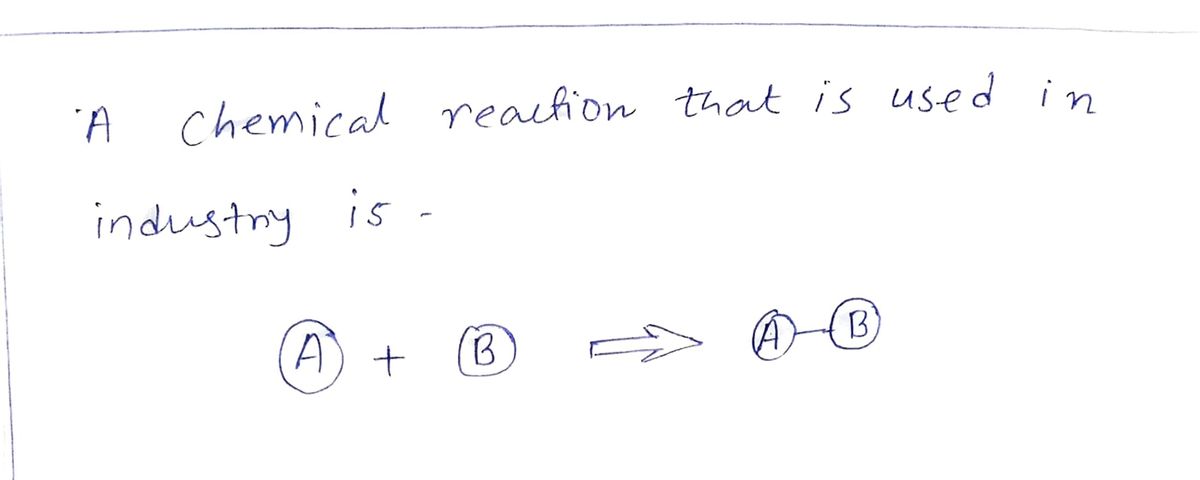
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning