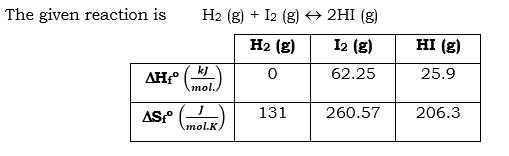
### Thermochemistry Question **Objective:** Calculate ΔG° for the formation of HI at 25°C using provided thermochemical data. #### Reaction: The formation of hydrogen iodide (HI) is given by the reaction: \[ \text{H}_2(g) + \text{I}_2(g) \rightarrow 2\text{HI}(g) \] #### Data Table: The table below provides enthalpy and entropy values for the substances in the reaction: | | \(\text{H}_2(g)\) | \(\text{I}_2(g)\) | \(\text{HI}(g)\) | |-----|----------------|----------------|--------------| | \(\Delta H^\circ_f \, (\text{kJ/mol})\) | 0 | 62.25 | 25.9 | | \(\Delta S^\circ_f \, (\text{J/mol})\) | 131 | 260.57 | 206.3 | #### Calculation: Use the Gibbs free energy equation to find \(\Delta G^\circ_\text{rxn}\): \[ \Delta G^\circ_\text{rxn} = \Delta H^\circ_\text{rxn} - T \Delta S^\circ_\text{rxn} \] Make sure to convert units where necessary (e.g., entropy from J/mol to kJ/mol). #### Equation: Enter your answer in kJ/mol in the space provided: \[\Delta G^\circ_\text{rxn} = \boxed{\phantom{\rule{2cm}{0.4pt}}} \, \text{kJ/mol}\] Click "Next part" to continue with the exercise. **Note:** Ensure calculations are thorough and check unit consistency.
### Thermochemistry Question **Objective:** Calculate ΔG° for the formation of HI at 25°C using provided thermochemical data. #### Reaction: The formation of hydrogen iodide (HI) is given by the reaction: \[ \text{H}_2(g) + \text{I}_2(g) \rightarrow 2\text{HI}(g) \] #### Data Table: The table below provides enthalpy and entropy values for the substances in the reaction: | | \(\text{H}_2(g)\) | \(\text{I}_2(g)\) | \(\text{HI}(g)\) | |-----|----------------|----------------|--------------| | \(\Delta H^\circ_f \, (\text{kJ/mol})\) | 0 | 62.25 | 25.9 | | \(\Delta S^\circ_f \, (\text{J/mol})\) | 131 | 260.57 | 206.3 | #### Calculation: Use the Gibbs free energy equation to find \(\Delta G^\circ_\text{rxn}\): \[ \Delta G^\circ_\text{rxn} = \Delta H^\circ_\text{rxn} - T \Delta S^\circ_\text{rxn} \] Make sure to convert units where necessary (e.g., entropy from J/mol to kJ/mol). #### Equation: Enter your answer in kJ/mol in the space provided: \[\Delta G^\circ_\text{rxn} = \boxed{\phantom{\rule{2cm}{0.4pt}}} \, \text{kJ/mol}\] Click "Next part" to continue with the exercise. **Note:** Ensure calculations are thorough and check unit consistency.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![### Thermochemistry Question
**Objective:** Calculate ΔG° for the formation of HI at 25°C using provided thermochemical data.
#### Reaction:
The formation of hydrogen iodide (HI) is given by the reaction:
\[ \text{H}_2(g) + \text{I}_2(g) \rightarrow 2\text{HI}(g) \]
#### Data Table:
The table below provides enthalpy and entropy values for the substances in the reaction:
| | \(\text{H}_2(g)\) | \(\text{I}_2(g)\) | \(\text{HI}(g)\) |
|-----|----------------|----------------|--------------|
| \(\Delta H^\circ_f \, (\text{kJ/mol})\) | 0 | 62.25 | 25.9 |
| \(\Delta S^\circ_f \, (\text{J/mol})\) | 131 | 260.57 | 206.3 |
#### Calculation:
Use the Gibbs free energy equation to find \(\Delta G^\circ_\text{rxn}\):
\[
\Delta G^\circ_\text{rxn} = \Delta H^\circ_\text{rxn} - T \Delta S^\circ_\text{rxn}
\]
Make sure to convert units where necessary (e.g., entropy from J/mol to kJ/mol).
#### Equation:
Enter your answer in kJ/mol in the space provided:
\[\Delta G^\circ_\text{rxn} = \boxed{\phantom{\rule{2cm}{0.4pt}}} \, \text{kJ/mol}\]
Click "Next part" to continue with the exercise.
**Note:** Ensure calculations are thorough and check unit consistency.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fcb9e9c53-4f67-4b94-a326-480d7802c16b%2F34ac7d64-657f-49bd-a895-07c9dbf40264%2Ftb0mp4g_processed.jpeg&w=3840&q=75)
Transcribed Image Text:### Thermochemistry Question
**Objective:** Calculate ΔG° for the formation of HI at 25°C using provided thermochemical data.
#### Reaction:
The formation of hydrogen iodide (HI) is given by the reaction:
\[ \text{H}_2(g) + \text{I}_2(g) \rightarrow 2\text{HI}(g) \]
#### Data Table:
The table below provides enthalpy and entropy values for the substances in the reaction:
| | \(\text{H}_2(g)\) | \(\text{I}_2(g)\) | \(\text{HI}(g)\) |
|-----|----------------|----------------|--------------|
| \(\Delta H^\circ_f \, (\text{kJ/mol})\) | 0 | 62.25 | 25.9 |
| \(\Delta S^\circ_f \, (\text{J/mol})\) | 131 | 260.57 | 206.3 |
#### Calculation:
Use the Gibbs free energy equation to find \(\Delta G^\circ_\text{rxn}\):
\[
\Delta G^\circ_\text{rxn} = \Delta H^\circ_\text{rxn} - T \Delta S^\circ_\text{rxn}
\]
Make sure to convert units where necessary (e.g., entropy from J/mol to kJ/mol).
#### Equation:
Enter your answer in kJ/mol in the space provided:
\[\Delta G^\circ_\text{rxn} = \boxed{\phantom{\rule{2cm}{0.4pt}}} \, \text{kJ/mol}\]
Click "Next part" to continue with the exercise.
**Note:** Ensure calculations are thorough and check unit consistency.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY