**Title: Understanding the Decomposition of Hydrogen Peroxide** **Question #18:** What is the rate law for the decomposition of hydrogen peroxide in an aqueous solution at 20°C? --- **Data Analysis:** The following data have been obtained for the decomposition reaction: \[ \text{2H}_2\text{O}_2(aq) \rightarrow 2\text{H}_2\text{O}(l) + \text{O}_2(g) \] | Time (min) | [H\(_2\)O\(_2\)] M (mol/L) | |------------|-----------------------------| | 0 | 9.83 \times 10^{-3} | | 3 | 8.64 \times 10^{-3} | | 6 | 6.03 \times 10^{-3} | | 9 | 3.64 \times 10^{-3} | | 12 | 2.53 \times 10^{-3} | | 15 | 1.52 \times 10^{-3} | | 18 | 0.76 \times 10^{-3} | | 21 | 0 | --- **Discussion:** The average rate of decomposition of hydrogen peroxide at 20°C over the first time interval (0 to 3 min) is calculated by the change in concentration of H\(_2\)O\(_2\) over that time. Notably, this data can help determine the half-life and rate constant of the reaction under controlled conditions at 20°C. --- Note: This information provides an example of analyzing reaction kinetics in a laboratory setting, emphasizing the importance of time and concentration in understanding chemical processes.
**Title: Understanding the Decomposition of Hydrogen Peroxide** **Question #18:** What is the rate law for the decomposition of hydrogen peroxide in an aqueous solution at 20°C? --- **Data Analysis:** The following data have been obtained for the decomposition reaction: \[ \text{2H}_2\text{O}_2(aq) \rightarrow 2\text{H}_2\text{O}(l) + \text{O}_2(g) \] | Time (min) | [H\(_2\)O\(_2\)] M (mol/L) | |------------|-----------------------------| | 0 | 9.83 \times 10^{-3} | | 3 | 8.64 \times 10^{-3} | | 6 | 6.03 \times 10^{-3} | | 9 | 3.64 \times 10^{-3} | | 12 | 2.53 \times 10^{-3} | | 15 | 1.52 \times 10^{-3} | | 18 | 0.76 \times 10^{-3} | | 21 | 0 | --- **Discussion:** The average rate of decomposition of hydrogen peroxide at 20°C over the first time interval (0 to 3 min) is calculated by the change in concentration of H\(_2\)O\(_2\) over that time. Notably, this data can help determine the half-life and rate constant of the reaction under controlled conditions at 20°C. --- Note: This information provides an example of analyzing reaction kinetics in a laboratory setting, emphasizing the importance of time and concentration in understanding chemical processes.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Title: Understanding the Decomposition of Hydrogen Peroxide**
**Question #18:**
What is the rate law for the decomposition of hydrogen peroxide in an aqueous solution at 20°C?
---
**Data Analysis:**
The following data have been obtained for the decomposition reaction:
\[ \text{2H}_2\text{O}_2(aq) \rightarrow 2\text{H}_2\text{O}(l) + \text{O}_2(g) \]
| Time (min) | [H\(_2\)O\(_2\)] M (mol/L) |
|------------|-----------------------------|
| 0 | 9.83 \times 10^{-3} |
| 3 | 8.64 \times 10^{-3} |
| 6 | 6.03 \times 10^{-3} |
| 9 | 3.64 \times 10^{-3} |
| 12 | 2.53 \times 10^{-3} |
| 15 | 1.52 \times 10^{-3} |
| 18 | 0.76 \times 10^{-3} |
| 21 | 0 |
---
**Discussion:**
The average rate of decomposition of hydrogen peroxide at 20°C over the first time interval (0 to 3 min) is calculated by the change in concentration of H\(_2\)O\(_2\) over that time. Notably, this data can help determine the half-life and rate constant of the reaction under controlled conditions at 20°C.
---
Note: This information provides an example of analyzing reaction kinetics in a laboratory setting, emphasizing the importance of time and concentration in understanding chemical processes.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F0eddc0f7-2e7e-4a89-95e7-969c8709c3f4%2F268fa6b7-f0fb-4410-9edb-903e7e56cad5%2F3lk9ffm.jpeg&w=3840&q=75)
Transcribed Image Text:**Title: Understanding the Decomposition of Hydrogen Peroxide**
**Question #18:**
What is the rate law for the decomposition of hydrogen peroxide in an aqueous solution at 20°C?
---
**Data Analysis:**
The following data have been obtained for the decomposition reaction:
\[ \text{2H}_2\text{O}_2(aq) \rightarrow 2\text{H}_2\text{O}(l) + \text{O}_2(g) \]
| Time (min) | [H\(_2\)O\(_2\)] M (mol/L) |
|------------|-----------------------------|
| 0 | 9.83 \times 10^{-3} |
| 3 | 8.64 \times 10^{-3} |
| 6 | 6.03 \times 10^{-3} |
| 9 | 3.64 \times 10^{-3} |
| 12 | 2.53 \times 10^{-3} |
| 15 | 1.52 \times 10^{-3} |
| 18 | 0.76 \times 10^{-3} |
| 21 | 0 |
---
**Discussion:**
The average rate of decomposition of hydrogen peroxide at 20°C over the first time interval (0 to 3 min) is calculated by the change in concentration of H\(_2\)O\(_2\) over that time. Notably, this data can help determine the half-life and rate constant of the reaction under controlled conditions at 20°C.
---
Note: This information provides an example of analyzing reaction kinetics in a laboratory setting, emphasizing the importance of time and concentration in understanding chemical processes.
Expert Solution
Step 1
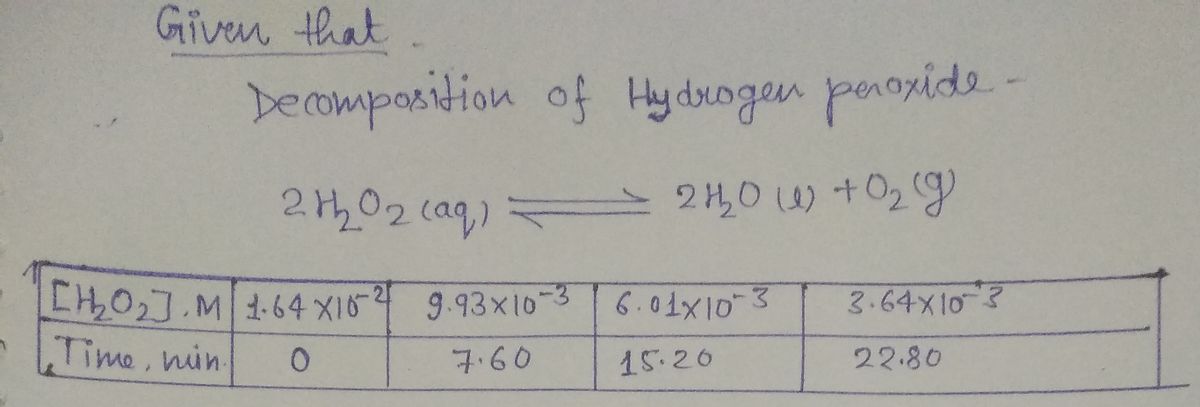
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY