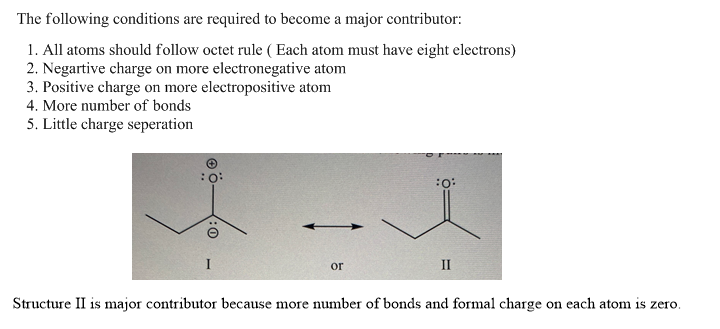
**Resonance Structures Analysis** The image presents three pairs of resonance structures that need to be analyzed to determine which one in each pair is more stable. Here is a detailed breakdown: ### Pair 1: - **Structure I**: - Composition: A carbonyl group adjacent to a CH2 group. The oxygen atom has a full negative charge (represented by "-") and the carbon atom bonded to CH2 has a positive charge (represented by "+"). - Arrangement: The electrons are in the form of a lone pair on oxygen, and there's a double bond between the carbon and oxygen. - **Structure II**: - Composition: Similar to Structure I, but with a double bond between the carbon and the adjacent CH2 group. The negative charge is on a different atom compared to Structure I. ### Pair 2: - **Structure I**: - Composition: A carbonyl group adjacent to a CH3 group. The oxygen atom carries a negative charge, and the carbon connected to CH3 has a positive charge. - Arrangement: Double bond between carbon and oxygen. - **Structure II**: - Composition: Similar atom arrangement to Structure I, but the location of double bonds and charges differ. Double bond appears between the carbon and CH3. ### Pair 3: - **Structure I**: - Composition: A carbonyl group attached directly without additional substituents. - Arrangement: Negative charge on the oxygen; no positive charge depicted. - **Structure II**: - Composition: Similar to Structure I with a focus on the resonance effect between atoms. - Arrangement: Lone pairs on oxygen, but lacks positive charge details. **Question Prompt**: "Which resonance structure in each of the following pairs is more stable? Why?" **Analysis Strategy**: To determine stability: - Consider resonance criteria: the distribution of charges, electron delocalization, and neutral charge preference. - Structures often stabilize by delocalizing charges and preferring configurations without charges or with full octets. Students should evaluate each pair based on these principles to identify the more stable structure and justify their rationale.
**Resonance Structures Analysis** The image presents three pairs of resonance structures that need to be analyzed to determine which one in each pair is more stable. Here is a detailed breakdown: ### Pair 1: - **Structure I**: - Composition: A carbonyl group adjacent to a CH2 group. The oxygen atom has a full negative charge (represented by "-") and the carbon atom bonded to CH2 has a positive charge (represented by "+"). - Arrangement: The electrons are in the form of a lone pair on oxygen, and there's a double bond between the carbon and oxygen. - **Structure II**: - Composition: Similar to Structure I, but with a double bond between the carbon and the adjacent CH2 group. The negative charge is on a different atom compared to Structure I. ### Pair 2: - **Structure I**: - Composition: A carbonyl group adjacent to a CH3 group. The oxygen atom carries a negative charge, and the carbon connected to CH3 has a positive charge. - Arrangement: Double bond between carbon and oxygen. - **Structure II**: - Composition: Similar atom arrangement to Structure I, but the location of double bonds and charges differ. Double bond appears between the carbon and CH3. ### Pair 3: - **Structure I**: - Composition: A carbonyl group attached directly without additional substituents. - Arrangement: Negative charge on the oxygen; no positive charge depicted. - **Structure II**: - Composition: Similar to Structure I with a focus on the resonance effect between atoms. - Arrangement: Lone pairs on oxygen, but lacks positive charge details. **Question Prompt**: "Which resonance structure in each of the following pairs is more stable? Why?" **Analysis Strategy**: To determine stability: - Consider resonance criteria: the distribution of charges, electron delocalization, and neutral charge preference. - Structures often stabilize by delocalizing charges and preferring configurations without charges or with full octets. Students should evaluate each pair based on these principles to identify the more stable structure and justify their rationale.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Resonance Structures Analysis**
The image presents three pairs of resonance structures that need to be analyzed to determine which one in each pair is more stable. Here is a detailed breakdown:
### Pair 1:
- **Structure I**:
- Composition: A carbonyl group adjacent to a CH2 group. The oxygen atom has a full negative charge (represented by "-") and the carbon atom bonded to CH2 has a positive charge (represented by "+").
- Arrangement: The electrons are in the form of a lone pair on oxygen, and there's a double bond between the carbon and oxygen.
- **Structure II**:
- Composition: Similar to Structure I, but with a double bond between the carbon and the adjacent CH2 group. The negative charge is on a different atom compared to Structure I.
### Pair 2:
- **Structure I**:
- Composition: A carbonyl group adjacent to a CH3 group. The oxygen atom carries a negative charge, and the carbon connected to CH3 has a positive charge.
- Arrangement: Double bond between carbon and oxygen.
- **Structure II**:
- Composition: Similar atom arrangement to Structure I, but the location of double bonds and charges differ. Double bond appears between the carbon and CH3.
### Pair 3:
- **Structure I**:
- Composition: A carbonyl group attached directly without additional substituents.
- Arrangement: Negative charge on the oxygen; no positive charge depicted.
- **Structure II**:
- Composition: Similar to Structure I with a focus on the resonance effect between atoms.
- Arrangement: Lone pairs on oxygen, but lacks positive charge details.
**Question Prompt**: "Which resonance structure in each of the following pairs is more stable? Why?"
**Analysis Strategy**:
To determine stability:
- Consider resonance criteria: the distribution of charges, electron delocalization, and neutral charge preference.
- Structures often stabilize by delocalizing charges and preferring configurations without charges or with full octets.
Students should evaluate each pair based on these principles to identify the more stable structure and justify their rationale.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY